Some aspects of hydrogen oxidation in solid oxide fuel cell: A brief historical overview
Abstract
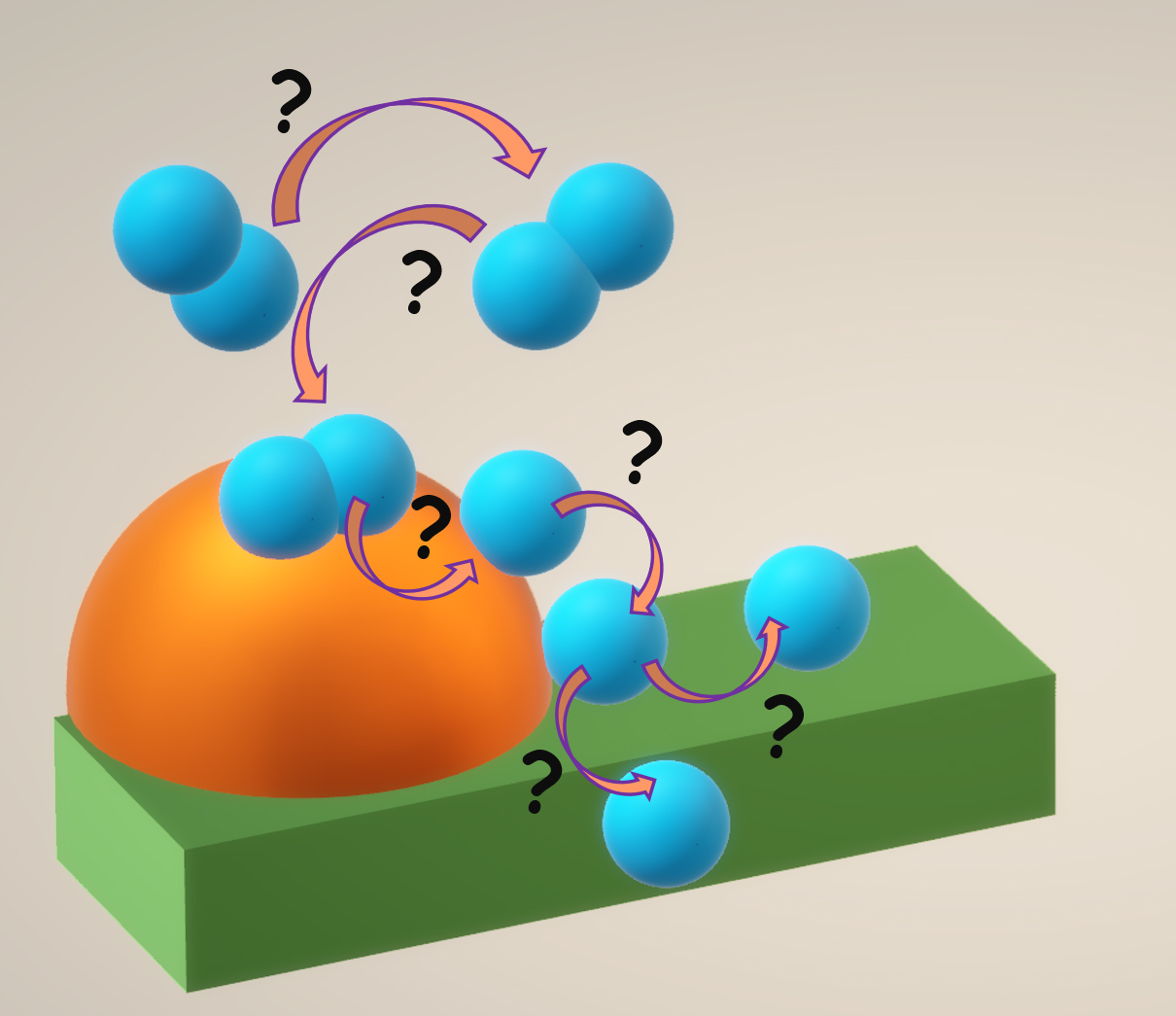
Environmentally friendly and resource-efficient ways to generate, convert, store and transport electricity are important areas of scientific and technological development. Fuel cells are direct converters of chemical energy into electricity with low emissions of harmful components. One of the most promising types of fuel cells is the solid oxide fuel cell (SOFC). The electrical power generated by the SOFC is mainly limited by the ohmic resistance of the electrolyte and the polarization of the electrodes. The ohmic resistance can be reduced by reducing the thickness of the electrolyte. To reduce the polarization resistance, other approaches are needed, namely a detailed study of the mechanisms of electrode reactions and the determination of the nature of rate-determining stages. Until now, fuel oxidation at the anode of the SOFC, as opposed to oxygen reduction at the cathode, has not been well understood. Even for conventional nickel-ceramic anodes, there is no clear understanding of the nature of the rate-determining steps of hydrogen oxidation. This review provides a brief historical background on the development of SOFCs, some insights into the oxygen reduction mechanisms, and a more detailed review of the kinetics of hydrogen oxidation at SOFC anodes.
Keywords
Full Text:
PDFReferences
Schottky W, Über stromliefernde Prozesse im Konzentrationsgefälle fester Elektrolyte, Wiss. Veröff. Siemens-Werken, 14 (2) (1935) 1–19.
Nernst W, assignor to George Westinghouse, of Pittsburg, Pennsylvania, Electrical glow light. United States patent US623811A. 1899 Apr 25.
Nernst W, Uber die elektrolvtische Leitung Fester Korper bei sehr hohen Temperaturen, Z. Elektrochem. 6 (2) (1899) 41-43. https://doi.org/10.1002/bbpc.18990060205
Bauer E, Brunner R, Uber die Eisenoxyd-Kathode in der Kohle-Luft-Kette, Z, Elektrochem. 43 (9) (1937) 725–727. https://doi.org/10.1002/bbpc.19370430902
Bauer E, Preis H, Uber Brennstoff-Ketten mit Festleitern, Z, Elektrochem. 43 (9) (1937) 727–732. https://doi.org/10.1002/bbpc.19370430903
Wagner C, Über den mechanismus der elektrischen Stromleitung im Nernststift, Naturwissenschaften, 31 (1943) 265–268. https://doi.org/10.1007/BF01475685
Weissbart J, Ruka R, A solid electrolyte fuel cell, J. Electrochem. Soc. 109 (1962) 723–726. https://doi.org/10.1149/1.2425537
Zhu B, Mi Y, Xia C, Wang B, et al., A nanoscale perspective on solid oxide and semiconductor membrane fuel cells: materials and technology, Energy Mater. 1 (2021) 100002. https://doi.org/10.20517/energymater.2021.03
Filonova E, Pikalova E, Overview of Approaches to Increase the Electrochemical Activity of Conventional Perovskite Air Electrodes, Materials, 16 (14) (2023) 4967. https://doi.org/10.3390/ma16144967
Porotnikova N, Osinkin D, Recent advances in heteroatom substitution Sr2Fe1.5Mo0.5O6–δ oxide as a more promising electrode material for symmetrical solid-state electrochemical devices: A review, Electrochem. Mater. Technol. 1 (2022) 20221003. https://doi.org/10.15826/elmattech.2022.1.003
Saheli B, Gurpreet K, Gary P, Sarbjit G, A critical review on cathode materials for steam electrolysis in solid oxide electrolysis, Int. J. Hydrogen Energy, 48 (34) (2023) 12541–12570. https://doi.org/10.1016/j.ijhydene.2022.11.307
Hu S, Li J, Zeng Y, Pu J, et al., A mini review of the recent progress of electrode materials for low-temperature solid oxide fuel cells, Phys. Chem. Chem. Phys. 25 (2023) 5926–5941. https://doi.org/10.1039/D2CP05133H
Pikalova EY, Kalinina EG, Pikalova NS, Filonova EA, High-Entropy Materials in SOFC Technology: Theoretical Foundations for Their Creation, Features of Synthesis, and Recent Achievements, Materials, 15 (24) (2022) 8783. https://doi.org/10.3390/ma15248783
Setoguchi T, Okamoto K, Eguchi K, Arai H, Effects of anode material and fuel on anodic reaction of solid oxide fuel cells, J. Electrochem. Soc. 139(10) (1992) 2875–2880. https://doi.org/10.1149/1.2068998
Rossmeisl J, Bessler WG, Trends in catalytic activity for SOFC anode materials, Solid State Ionics, 178 (2008) 1694–1700. https://doi.org/10.1016/j.ssi.2007.10.016
Lee DS, Lee JH, Kim J, Lee HW, et al., Tuning of the microstructure and electrical properties of SOFC anode via compaction pressure control during forming, Solid State Ionics, 166 (2004) 13–17. https://doi.org/10.1016/j.ssi.2003.10.003
Corbin SF, Qiao X, Development of Solid Oxide Fuel Cell Anodes Using Metal-Coated Pore-Forming Agents, J. Am. Ceram. Soc. 86 (2003) 401–406. https://doi.org/10.1111/j.1151-2916.2003.tb03312.x
Koide H, Someya Y, Yoshida T, Maruyama T, Properties of Ni/YSZ cermet as anode for SOFC, Solid State Ionics, 132 (2000) 253–260. https://doi.org/10.1016/S0167-2738(00)00652-4
Osinkin DA, Bronin DI, Beresnev SM, Bogdanovich NM, et al., Thermal expansion, gas permeability, and conductivity of Ni-YSZ anodes produced by different techniques, J. Solid State Electrochem. 18 (2014) 149–156. https://doi.org/10.1007/s10008-013-2239-4
Osinkin DA, Long-term tests of Ni-Zr0.9Sc0.1O1.95 anode impregnated with CeO2 in H2 + H2O gas mixtures, Int. J. Hydrogen Energy, 41 (2016) 17577–17584. http://doi.org/10.1016/j.ijhydene.2016.07.136
Osinkin DA, Degradation of Ni-Zr0.9Sc0.1O1.95 anode in H2 + H2O at low temperature: Influence of nickel surface charge, Int. J. Hydrogen Energy, 43 (2018) 943–950. https://doi.org/10.1016/j.ijhydene.2017.11.071
Feng M, Goodenough JB, Huang K, Milliken C, Fuel cells with doped lanthanum gallate electrolyte, J. Power Sources, 63 (1996) 47–51. https://doi.org/10.1016/S0378-7753(96)02441-X
Huang K, Tichy R, Goodenough JB, Superior Perovskite Oxide‐Ion Conductor; Strontium‐ and Magnesium‐Doped LaGaO3: I. Phase Relationships and Electrical Properties, J. Am. Ceram. Soc. 81 (1998) 2565–2575. https://doi.org/10.1111/j.1151-2916.1998.tb02662.x
Hua D, Li G, Li H, Zhang X, et al., Investigation of carbon formation on Ni/YSZ anode of solid oxide fuel cell from CO disproportionation reaction, Int. Comm. Heat and Mass Tran. 91 (2018) 23–29. https://doi.org/10.1016/j.icheatmasstransfer.2017.11.014
Jia L, Wang X, Hua B, Li W, et al., Computational analysis of atomic C and S adsorption on Ni, Cu, and Ni-Cu SOFC anode surfaces, Int. J. Hydrogen Energy, 37 (2012) 11941–11945. https://doi.org/10.1016/j.ijhydene.2012.05.041
Ye X, Zhou J, Zeng SR, Wen TL, et al., Research of carbon deposition formation and judgment in Cu-CeO2-ScSZ anodes for direct ethanol solid oxide fuel cells, Int. J. Hydrogen Energy, 37 (2012) 505–510. https://doi.org/10.1016/j.ijhydene.2011.09.017
An S, Lu C, Worrell WL, Vohs JM, Characterization of Cu–CeO2 direct hydrocarbon anodes in a solid oxide fuel cell with lanthanum gallate electrolyte, Solid State Ionics, 175 (2004) 135–138. https://doi.org/10.1016/j.ssi.2004.09.029
Kolotygin V. Materiais a Base de Oxidos com Estrutura do Tipo Perovskite e Compositos como Anodos de PCES Propriedades Funcionais e Comportamento Eletroquimico em Celulas com Eletrolitos Solidos a Base de Galatos e Silicatos [PhD Thesis]. Aveiro (Portugal): Universidade de Aveiro, Portugal; 2015. 259 p.
Fu QX, Tietz F, Stover D, La0.4Sr0.6Ti1-xMnxO3–δ perovskites as anode materials for solid oxide fuel cells, J. Electrochem. Soc. 153 (2006) D74–D83. https://doi.org/10.1149/1.2170585
Azad AK, Eriksson GG, Irvine JTS, Structural, magnetic and electrochemical characterization of La0.83A0.17Fe0.5Cr0.5O3–δ (A = Ba, Ca) perovskites, Mater. Res. Bull. 44 (2009) 1451−1457. https://doi.org/10.1016/j.materresbull.2009.03.008
Deleebeck L, Fournier JL, Birss V, Comparison of Sr-doped and Sr-free La1–xSrxMn0.5Cr0.5O3±δ SOFC Anodes, Solid State Ionics, 181 (2010) 1229–1237. https://doi.org/10.1016/j.ssi.2010.05.027
Tao SW, Irvine JTS, Phase Transition in Perovskite Oxide La0.75Sr0.25Cr0.5Mn0.5O3–δ Observed by in Situ High-Temperature Neutron Powder Diffraction, Chem. Mater. 18 (2006) 5453–5460. https://doi.org/10.1021/cm061413n
Cascos V, Troncoso L, Alonso JA, Fernandez-Diaz MT, Design of new Ga-doped SrMoO3 perovskites performing as anode materials in SOFC, Renew. Energy, 111 (2017) 476–483. https://doi.org/10.1016/j.renene.2017.04.023
Childs NB, Wesinstein A, Smith R, Sofie S, et al., Key Electrical conductivity of Sr2–xVMoO6–y (x = 0.0, 0.1, 0.2) double perovskites, J. Appl. Phys. 113 (2013) 243506. https://doi.org/10.1063/1.4811715
Zamudio-García J, Caizan-Juanarena L, Porras-Vazquez JM, Losilla ER, Marrero-Lopez D, A review on recent advances and trends in symmetrical electrodes for solid oxide cells, J. Power Sources, 520 (2022) 230852. https://doi.org/10.1016/j.jpowsour.2021.230852
Zhu K, Luo B, Liu Z, Wen X, Recent advances and prospects of symmetrical solid oxide fuel cells, Ceram. Int. 48 (2022) 8972–8986. https://doi.org/10.1016/j.ceramint.2022.01.258
Ruiz-Morales JC, Marrero-Lo´pez D, Canales-Va´zquezc J, Irvine JTS, Symmetric and reversible solid oxide fuel cells, RSC Advances, 1 (2011) 1403–1414. https://doi.org/10.1039/C1RA00284H
Shu L, Sunarso J, Hashim SS, Mao J, et al., Advanced perovskite anodes for solid oxide fuel cells: A review, Int. J. Hydrogen Energy, 44 (2019) 31275–31304. https://doi.org/10.1016/j.ijhydene.2019.09.220
Hu H, Liu M, Interfacial polarization characteristics of Pt|BaCe0.8Gd0.2O3|Pt cells at intermediate temperatures, J. Electrochem. Soc. 144 (1997) 3561–3567. https://doi.org/10.1149/1.1838048
Antonova EP, Bronin DI, Stroeva AYu, Polarization resistance of platinum electrodes in contact with proton-conducting La0.9Sr0.1ScO3–δ, Russ. J. Electrochem. 50 (2014) 613–616. https://doi.org/10.1134/S1023193514070027
Liu M, Hu H, Effect of interfacial resistance on determination of transport properties of mixed-conducting electrolytes, J. Electrochem. Soc. 143 (6) (1996) L109–L112. https://doi.org/10.1149/1.1836892
Poetzsch D, Merkle R, Maier J, Investigation of oxygen exchange kinetics in proton-conducting ceramic fuel cells: Effect of electronic leakage current using symmetric cells, J. Power Sources, 242 (2013) 784–789. https://doi.org/10.1016/j.jpowsour.2013.05.108
Antonova EP, Electro transfer and kinetics of electrode processes in systems with proton conducting electrolytes with perovskite structure [dissertation]. Yekaterinburg (Russia): Institute of High Temperature Electrochemistry; 2015. 132 p.
Murygin IV. Electrodnye process v tverdyh electrolitah [Electrode processes in solid electrolytes]. Nauka: Moskva; 1991. 350 p. Russian.
Fabry P, Kleitz M, Influence of the metal and the electrolyte composition on the characteristics of the oxygen electrode reaction on solid oxide electrolyte, J. Electroanal. Chem. Inter. Electrochem. 57 (1974) 165-177. https://doi.org/10.1016/S0022-0728(74)80020-3
Perfiliev MV, Palguev SF. K voprosy o kinetike electrodnyh processov v tverdih electrolitah [Toward a question on the kinetics of electrode processes in solid electrolytes]. Collection of scientific papers of the Institute of Electrochemistry, UFAN USSR. 1966. 157–167.
Wang DY, Nowick AS, Diffusion‐Controlled Polarization of Pt, Ag, and Au Electrodes with Doped Ceria Electrolyte, J. Electrochem. Soc. 128 (1981) 55–63. https://doi.org/10.1149/1.2127387
Adler SB, Factors governing oxygen reduction in solid oxide fuel cell cathodes, Chem. Rev. 104 (2004) 4791–4844. https://doi.org/10.1021/cr020724o
Jiang SP, Activation, microstructure, and polarization of solid oxide fuel cell cathodes, J. Solid State Electrochem. 11 (2007) 93–102. https://doi.org/10.1007/s10008-005-0076-9
Steele BCH, Behaviour of porous cathodes in high temperature fuel cells, Solid State Ionics, 94 (1997) 239–248. https://doi.org/10.1016/S0167-2738(96)00510-3
Simner SP, Anderson MD, Pederson LR, Stevenson JW, Performance Variability of La(Sr)FeO3 SOFC Cathode with Pt, Ag, and Au Current Collectors, J. Electrochem. Soc. 152 (2005) A1851–A1859. https://doi.org/10.1149/1.1995687
Kinoshita K. Electrochemical oxygen technology: Wiley-Interscience; New York. 1992. 448 p.
Bouwmeester HJN, Burgraaf AJ. Dense ceramic membranes for oxygen separation In: Burggraaf AJ, Cot L (eds) Fundamentals of inorganic membrane science and technology. Elsevier: Amsterdam; 1996. 435–528.
Karpachev SV, Filyaev AT, K voprosy electrohimicheskoy kinetiki v slychae tverdogo electrolita [Toward the question of electrochemical kinetics in the case of solid electrolyte, Electrohimiya, 2 (11) (1966) 1330–1332.
Schouler E, Giroud G, Kleitz M, Applications selon Bauerle du trace des diagrammes d’admittance complex en électrochimie des solides. II. Étude de la conductivite de la zircone stabilisee a l’yttrium, J. Chim. Physiq. 70 (1973) 1309–1316. https://doi.org/10.1051/jcp/1973701309
Verkerk MJ, Burggraaf AJ, Oxygen transfer on substituted ZrO2, Bi2O3, and CeO2 electrolytes with platinum electrodes. II. a-c impedance study, J. Electrochem. Soc. 130 (1) (1983) 78–84. https://doi.org/10.1149/1.2119687
Verkerk MJ, Hammink MWJ, Burggraaf AJ, Oxygen transfer on substituted ZrO2, Bi2O3, and CeO2 electrolytes with platinum electrodes. I. Electrode resistance by d-c polarization, J. Electrochem. Soc. 130 (1) (1983) 70–78. https://doi.org/10.1149/1.2119686
Bauerle JE, Study of solid electrolyte polarization by a complex admittance method, J. Phys. Chem. Solid. 30 (12) (1969) 2657–2670. https://doi.org/10.1016/0022-3697(69)90039-0
Mizusaki J, Amano K, Yamauchi S, Fueki K, Electrode reaction at Pt, O2(g)/stabilized zirconia interfaces. Part I: Theoretical consideration of reaction model, Solid State Ionics, 22 (1987) 313–322. https://doi.org/10.1016/0167-2738(87)90149-4
Mizusaki J, Amano K, Yamauchi S, Fueki K, Electrode reaction at Pt, O2(g)/stabilized zirconia interfaces. Part II: Electrochemical measurements and analysis, Solid State Ionics 22 (1987) 323–330. https://doi.org/10.1016/0167-2738(87)90150-0
Kuzin BL, Komarov MA, Adsorption of O2 at Pt and kinetics of the oxygen reaction at a porous Pt in contact with a solid oxide electrolyte, Solid State Ionics, 39 (1990) 163–172. https://doi.org/10.1016/0167-2738(90)90395-8
Wang DY, Nowick AS, Cathodic and anodic polarization phenomena at platinum electrodes with doped CeO2 as electrolyte. II. Transient overpotential and ac-impedance, J. Electrochem. Soc. 126 (7) (1979) 1166–1172. https://doi.org/10.1149/1.2129236
Winnubst AJA, Scharenborg AHA, Burggraaf AJ, The electrode resistance of ZrO2–Y2O3(–Bi2O3) solid electrolytes with Pt electrodes, Solid State Ionics, 14 (1984) 319–327. https://doi.org/10.1016/0167-2738(84)90116-4
Schwandt C, Weppner W, Kinetics of oxygen, platinum/stabilized zirconia and oxygen, gold/stabilized zirconia electrodes under equilibrium conditions, J. Electrochem. Soc. 144 (11) (1997) 3728–3738. https://doi.org/10.1149/1.1838083
Wang DY, Nowick AS, Cathodic and anodic polarization phenomena at platinum electrodes with doped CeO2 as electrolyte. I. Steady-state overpotential, J. Electrochem. Soc. 126 (7) (1979) 1155–1165. https://doi.org/10.1149/1.2129235
Sasaki J, Mizusaki H, Yamauchi S, Fueki K, Studies on electrode processes of stabilized zirconia systems by complex impedance method, Bull. Chem. Soc. Japan 54 (6) (1981) 1688–1692. https://doi.org/10.1246/bcsj.54.1688
Gür TM, Raistrick ID, Huggins RA, Steady-state d–c polarization characteristics of the O2, Pt/stabilized zirconia interface, J. Electrochem. Soc. 127 (12) (1980) 2620–2628. https://doi.org 10.1149/1.2129532
Vayenas CG, Michaels VN, On the stability limit of surface platinum oxide and its role in oscillation phenomena of platinum catalyzed oxidations, Surf. Sci. 120 (1) (1982) L405–L408. https://doi.org/10.1016/0039-6028(82)90265-5
Vayenas CG, Ioannides A, Bebelis S, Solid electrolyte cyclic voltammetry in situ investigation of catalyst surfaces, J. Catal. 129 (1) (1991) 67–87. https://doi.org/10.1016/0021-9517(91)90010-2
Yentekakis IV, Neophytides S, Vayenas CG, Solid electrolyte aided study of the mechanism of CO oxidation on polycrystalline platinum, J. Catal. 111 (1) (1988) 152–169. https://doi.org/10.1016/0021-9517(88)90074-7
Chao T, Walsh KJ, Fedkiw PS, Cyclic voltammetric study of the electrochemical formation of platinum oxide in a Pt/yttria-stabilized zirconia cell, Solid State Ionics, 47 (3–4) (1991) 277–283. https://doi.org/10.1016/0167-2738(91)90250-F
Kuzin BL, Komarov MA, Termodinamika kislorodnogo electrode iz poristoy platiny v kontakte s tverdim oksidnim electrolitom pri visokih davleniyah kisloroda [Thermodynamics of porous platinum oxygen electrode in contact with solid oxide electrolyte at high oxygen pressures] Electrohimiya, 29 (11) (1993) 1374–1379.
Kuzin BL, Komarov MA, Opredelenie nizhney granicy T, Po2-polya termodinamicheskih pokazaniy kislorodnogo electrode iz porispoy platiny v kontakte s electrolitom na osnove dioksida zirkoniya [Determination of the lower limit of T, Ro2-field thermodynamic readings of the oxygen electrode made of porous platinum in contact with zirconium dioxide electrolyte] Electrohimiya, 27 (9) (1991) 1128–1132.
Glumov MV, Polarization of porous platinum electrodes in a solid-electrolyte cell in oxygen atmosphere, Soviet electrochemistry, 22 (2) (1986) 207–211.
Bronin DI, Kinetics of electrode processes in electrochemical systems with solid oxide electrolytes [dissertation]. Yekaterinburg (Russia): Institute of high temperature electrochemistry; 2007. 283 p.
Perfiliev MV, Lobovikova NA, Vliyanie anodnoy polyarizacii na svoystva kontakta serebryanih electrodov s electrolitom ZrO2 + Y2O3 [Effect of anodic polarization on the contact properties of silver electrodes with ZrO2 + Y2O3 electrolyte] Electrohimiya, 20 (1984) 322–327.
Moghadam FK, Stevenson DA, Oxygen diffusion and solubility studies in Ag and Pt using ac impedance spectroscopy, J. Electrochem. Soc. 133 (7) (1986) 1329−1332. https://doi.org/10.1016/10.1149/1.2108864
Jimenez R, Kloidt T, Kleitz M, Reaction-zone expansion and mechanism of the O2, Ag/yttria-stabilized zirconia electrode reaction, J. Electrochem. Soc. 144 (2) (1997) 582–585. https://doi.org/10.1149/1.1837451
Kuo JH, Anderson HU, Sparlin DM, Oxidation-reduction behavior of undoped and Sr-doped LaMnO3: defect structure, electrical conductivity, and thermoelectric power, J. Solid State Chem. 87 (1990) 55–63. https://doi.org/10.1016/0022-4596(90)90064-5
Kamata H, Yonemura Y, Mizusaki J, Tagawa H, Naraya K, Sasamoto T, High temperature electrical properties of the perovskite-type oxide La1–xSrxMnO3–δ, J. Phys. Chem. Solid. 56 (7) (1995) 943–950. https://doi.org/10.1016/0022-3697(95)00019-4
Endo A, Fukunaga H, Wen C, Yamada K, Cathodic reaction mechanism of dense La0.6Sr0.4CoO3 and La0.81Sr0.09MnO3, Solid State Ionics, 135 (2000) 353–358. https://doi.org/10.1016/S0167-2738(00)00466-5
Mizusaki J, Saito T, Tagawa H, A chemical diffusion-controlled electrode reaction at the compact
La1–xSrxMnO3/stabilized zirconia interface in oxygen atmospheres, J. Electrochem. Soc. 143 (10) (1996) 3065–3073. https://doi.org/10.1149/1.1837165
Ioroi T, Hara T, Uchimoto Y, Ogumi Z, Takehara Z, Preparation of perovskite-type La1–xSrxMnO3 films by vapor-phase processes and their electrochemical properties, J. Electrochem. Soc. 145 (6) (1998) 1999–2004. https://doi.org/10.1149/1.1838589
Mizusaki J, Tagawa H, Tsuneyoshi K, Sawata A, Reaction kinetics and microstructure of the solid oxide fuel cells air electrode La0.6Ca0.4MnO3/YSZ, J. Electrochem. Soc. 138 (7) (1991) 1867–1975. https://doi.org/10.1149/1.2085891
Ostergard MJL, Mogensen M. Ac impedance study of the oxygen reduction mechanism on La1–xSrxMnO3 in solid oxide fuel cells, Electrochim. Acta 38 (14) (1993) 2015–2020. https://doi.org/10.1016/0013-4686(93)80334-V
van Heuveln FH, Bouwmeester HJM, Electrode properties of Sr-doped LaMnO3 on yttria-stabilized zirconia. II. Electrode kinetics, J. Electrochem. Soc. 144 (1) (1997) 134–140. https://doi.org/10.1149/1.1837375
Sasaki K, Wurth J-P, Gschwend R, Godickemeier M, Gauckler LJ, Microstructure-property relations of solid oxide fuel cell cathodes and current collectors, J. Electrochem. Soc. 143 (2) (1996) 530–543. https://doi.org/10.1149/1.1836476
Takeda Y, Kanno R, Noda M, Tomida Y, Yamamoto O, Cathodic polarization phenomena of perovskite oxide electrodes with stabilized zirconia, J. Electrochem. Soc. 134 (11) (1987) 2656–2661. https://doi.org/10.1149/1.2100267
Siebert E, Hammouche A, Kleitz M, Impedance spectroscopy analysis of La1–xSrxMnO3-yttria-stabilized zirconia electrode kinetics, Electrochim. Acta, 40 (11) (1995) 1741–1753. https://doi.org/10.1016/0013-4686(94)00361-4
Adler SB, Lane JA, Steele BCH, Electrode Kinetics of Porous Mixed Conducting Oxygen Electrodes, J. Electrochem. Soc. 143 (11) (1996) 3554−3564. https://doi.org/10.1149/1.1837252
Mitterdorfer A, Gauckler LJ, Identification of the reaction mechanism of the Pt, O2(g)|yttria-stabilized zirconia system: Part I: General framework, modelling, and structural investigation, Solid State Ionics, 117 (1999) 187–202. https://doi.org/10.1016/S0167-2738(98)00341-5
Mitterdorfer A, Gauckler LJ, Identification of the reaction mechanism of the Pt, O2(g)|yttria-stabilized zirconia system: Part II: Model implementation, parameter estimation, and validation, Solid State Ionics, 117 (1999) 203–217. https://doi.org/10.1016/S0167-2738(98)00340-3
Ni M, Leung MKH, Leung DYC, Micro-scale modelling of solid oxide fuel cells with micro-structurally graded electrodes, J. Power Sources, 168 (2007) 369–378. https://doi.org/10.1016/j.jpowsour.2007.03.005
Ananyev MV. Isotopic exchange of oxygen and hydrogen gas with oxide electrochemical materials [dissertation]. Yekaterinburg (Russia): Institute of high temperature electrochemistry; 2016. 391 p.
Gerischer H, Wechselstrompolarisation von Elektroden mit einem potentialbestimmenden Schritt beim Gleichgewichtspotential II, Z. Phys. Chem. 201 (1952) 55−67. https://doi.org/10.1515/zpch-1952-20105
Osinkin DA, Khodimchuk AV, Antonova EP, Bogdanovich NM, Understanding the oxygen reduction kinetics on Sr2–xFe1.5Mo0.5O6–δ: Influence of strontium deficiency and correlation with the oxygen isotopic exchange data, Solid State Ionics, 374 (2022) 115818. https://doi.org/10.1016/j.ssi.2021.115818
Almar L, Sz´asz J, Weber A, Ivers-Tiffee E, Oxygen Transport Kinetics of Mixed Ionic-Electronic Conductors by Coupling Focused Ion Beam Tomography and Electrochemical Impedance Spectroscopy, J. Electrochem. Soc. 164 (4) (2017) F289–F297. https://doi.org/10.1149/2.0851704jes
Zupnik AE, Perfiliev MV, Karpachev SV, Polyarizaciay processa electrohimicheskogo vosstanovleniya pariv void v yacheykah s tverdim electrolitom [Polarization of electrochemical reduction of water vapor in cells with solid electrolyte], Electrohimiya, 7 (8) (1971) 1163–1167.
Zupnik AE, Perfiliev MV, Karpachev SV, Impedance granicy razdela platinovih electrodov s tverdim electrolitom v atmosphere vodoroda I void [Impedance of the interface of platinum electrodes with solid electrolyte in hydrogen and water atmosphere], Electrohimiya, 7 (8) (1971) 1188–1191.
Zupnik AE, Perfiliev MV, Karpachev SV, Impedance granicy platinoviy electrode / tverdiy electrolit v usloviyah katodnoy polyarizacii [Impedance of platinum electrode/solid electrolyte interface under cathodic polarization conditions], Electrohimiya, 8 (11) (1972) 1639–1641.
Kuzin BL, Neuymin AD, Palguev SF, Anodnaya polarizaciya tonkih electrodov v kontakte s tverdim electrolitom v gazovoy srede H2+H2O perennogo sostava [Anodic polarization of thin nickel electrodes in contact with solid electrolyte in H2+H2O gas medium of variable composition], Electrohimiya, 9 (1) (1973) 17–22.
Schouler EJL, Kleitz M, Forest E, Fernandez E, Fabry P, Overpotential of H2-H2O, Ni/YSZ electrodes in steam electrolyzers, Solid State Ionics, 5 (1981) 559–562. https://doi.org/10.1016/0167-2738(81)90316-7
Fernandez E. Electrolyse de la vapeur d’eau: characteristique de la reaction cathodique et conductivities de la zircone stabilisee [Ph.D. Theses]. Grenoble [France]; 1980. 105 p.
Kuzin BL. Investigation of electrode process kinetics on nickel electrode in contact with solid oxide electrolyte in H2+H2O atmosphere [dissertation]. Sverdlovsk [USSR]: Institute of electrochemistry; 1977. 156 p.
Mizusaki J, Tagawa H, Saito T, Kamitani K, et al., Hashimoto Preparation of nickel pattern electrodes on YSZ and their electrochemical properties in H2-H2O atmospheres, J. Electrochem. Soc. 141 (8) (1994) 2129–2134. https://doi.org/10.1149/1.2055073
Nakagawa N, Sakurai H, Kondo K, Morimoto T, et al., Evaluation of the effective reaction zone at Ni(NiO)/zirconia anode by using an electrode with a novel structure, J. Electrochem. Soc. 142 (10) (1995) 3474–3479. https://doi.org/10.1149/1.2050007
Holtappels P, Vinke IC, de Haart LGJ, Stimming U, Reaction of hydrogen/water mixtures on nickel-zirconia cermet electrodes. II. Ac polarization characteristics, J. Electrochem. Soc. 146 (8) (1999) 2976–2982. https://doi.org/10.1149/1.1392038
de Boer B, Gonzalez M, Bowmeester HJM, Verweij H, The effect of the presence of fine YSZ particles on the performance of porous nickel electrodes, Solid State Ionics, 127 (2000) 269–276. https://doi.org/10.1016/S0167-2738(99)00299-4
Mogensen M, Skaarup S, Kinetic and geometric aspects of solid oxide fuel cell electrodes, Solid State Ionics, 86–88 (1996) 1151–1160. https://doi.org/10.1016/0167-2738(96)00280-9
Mogensen M, Lindegaard T. The kinetics of hydrogen oxidation on a Ni/YSZ SOFC electrode at 1000 ºC. In: 3rd International Symposium on Solid Oxide Fuel Cells (SOFC-III); 1993; Honolulu, HI, USA. 484–493.
de Boer B. SOFC Anode: hydrogen oxidation at porous nickel and nickel/yttria-stabilised zirconia cermet electrodes [Ph.D. thesis]. Enschede (Netherlands): University of Twente; 1998. 161 p.
Lee WY, Wee D, Ghoniem AF, An improved one-dimensional membrane electrode assembly model to predict the performance of solid oxide fuel cell including the limiting current density, J. Power Sources, 186 (2009) 417–427. https://doi.org/10.1016/j.jpowsour.2008.10.009
Jiang SP, Badwal SPS, An electrode kinetics study of H2 oxidation on Ni/Y2O3-ZrO2 cermet electrode of the solid oxide fuel cell, Solid State Ionics, 123 (1999) 209–224. https://doi.org/10.1016/S0167-2738(99)00124-1
Jiang SP, Badwal SPS, Hydrogen oxidation at the nickel and platinum electrodes on yttria-tetragonal zirconia electrolyte, J. Electrochem. Soc. 144 (11) (1997) 3777–3784. https://doi.org/10.1149/1.1838091
Bieberle A, Meier LP, Gauckler LJ, The electrochemistry of Ni pattern anodes used as solid oxide fuel cell model electrodes, J. Electrochem. Soc. 148 (6) (2001) A646–A656. https://doi.org/10.1149/1.1372219
Bieberle A. The electrochemistry of solid oxide fuel cell anodes: experiments, modeling, and simulation [Ph.D. thesis]. Zurich (Switzerland): Swiss Federal Institute of Technology; 2000. 222 p.
Bieberle A, Gauckler LJ, State-space modeling of the anodic SOFC system Ni, H2-H2O/YSZ, Solid State Ionics, 146 (2002) 23–41. https://doi.org/10.1016/S0167-2738(01)01004-9
Bessler WG, Warnatz J, Goodwin DG, The influence of equilibrium potential on the hydrogen oxidation kinetics of SOFC anodes, Solid State Ionics, 177 (2007) 3371–3383. https://doi.org/10.1016/j.ssi.2006.10.020
Jiang Y, Virkar AV, Fuel composition and diluent effects on gas transport and performance of anode-supported SOFCs, J. Electrochem. Soc. 150 (7) (2003) A942–A951. https://doi.org/10.1149/1.1579480
Matsuzaki Y, Yasuda I, Electrochemical oxidation of H2 and CO in a H2-H2O-CO-CO2 system at the interface of a Ni-YSZ cermet electrode and YSZ electrolyte, J. Electrochem. Soc. 147 (5) (2000) 1630–1635. https://doi.org/10.1149/1.1393409
Eguchi K, Kojo H, Takeguchi T, Kikuchi R, et al., Fuel flexibility in power generation by solid oxide fuel cells, Solid State Ionics, 152–153 (2002) 411–416. https://doi.org/10.1016/S0167-2738(02)00351-X
Sasaki K, Hori Y, Kikuchi R, Eguchi K, et al., Uchida Current-voltage characteristics and impedance analysis of solid oxide fuel cells for mixed H2 and CO gases, J. Electrochem. Soc. 149 (3) (2002) A227–A233. https://doi.org/10.1149/1.1435357
Etsell TH, Flengas SN, Overpotential behavior of stabilized zirconia solid electrolyte fuel cells, J. Electrochem. Soc. 118 (12) (1971) 1890–1900. https://doi.org/10.1149/1.2407862
Lauvstad GO, Tunold R, Sunde S, Electrochemical oxidation of CO on Pt and Ni point electrodes in contact at yttria-stabilized zirconia electrolyte, J. Electrochem. Soc. 149 (12) (2002) E497–E505. https://doi.org/10.1149/1.1518484
Somov SI, Perfiliev MV, Polyarizacionnye harakteristiki electrodnoy sistemy CO + CO2/M + CeO2–x/0.91ZrO2 + 0.09Y2O3 [Polarization characteristics of the electrode system CO + CO2/M + CeO2–x/0.91ZrO2 + 0.09Y2O3. In: Electrodnye reakcii v tverdyh electrolitah; 1990; Sverdlovsk, 80–90.
Zhu T, Fowler DE, Poeppelmeier KR, Han M, Barnett SA, Hydrogen Oxidation Mechanisms on Perovskite Solid Oxide Fuel Cell Anodes, J. Electrochem. Soc. 163 (8) (2016) F952–F961. https://doi.org/10.1149/2.1321608jes
Chen M, Chen D, Chang M, Hu H, Xu Q, New Insight into Hydrogen Oxidation Reaction on La0.3Sr0.7Fe0.7Cr0.3O3–δ Perovskite as a Solid Oxide Fuel Cell Anode, J. Electrochem. Soc. 164 (2017) F405–F411. https://doi.org/10.1149/2.1571704jes
Niu B, Jin F, Yang X, Feng T, He T, Resisting coking and sulfur poisoning of double perovskite Sr2TiFe0.5Mo0.5O6–δ anode material for solid oxide fuel cells, Int. J. Hydrogen Energy, 43 (6) (2018) 3280–3290. https://doi.org/10.1016/j.ijhydene.2017.12.134
Bian L, Duan C, Wang L, Zhu L, et al., Electrochemical performance and stability of La0.5Sr0.5Fe0.9Nb0.1O3–δ symmetric electrode for solid oxide fuel cells, J. Power Sources, 399 (2018) 398–405. https://doi.org/10.1016/j.jpowsour.2018.07.119
Niu B, Jin F, Fu R, Feng T, Shen Y, Liu J, He T, Pd-impregnated Sr1.9VMoO6–δ double perovskite as an efficient and stable anode for solid-oxide fuel cells operating on sulfur-containing syngas, Electrochim. Acta, 274 (2018) 91–102. https://doi.org/10.1016/j.electacta.2018.04.066
Ammal SC, Heyden A, Reaction kinetics of the electrochemical oxidationof CO and syngas fuels on a Sr2Fe1.5Mo0.5O6–δ perovskite anode, J. Mater. Chem. A, 3 (2015) 21618–21629. https://doi.org/10.1039/C5TA05056A
Yang Y, Li Y, Jiang Y, Zheng M, et al., The electrochemical performance and CO2 reduction mechanism on strontium doped lanthanum ferrite fuel electrode in solid oxide electrolysis cell, Electrochim. Acta, 284 (2018) 159–167. https://doi.org/10.1016/j.electacta.2018.07.187
Li J, Wei B, Wang C, Zhou Z, Lu Z, High-performance and stable La0.8Sr0.2Fe0.9Nb0.1O3–δ anode for direct carbon solid oxide fuel cells fueled by activated carbon and corn straw derived carbon, Int. J. Hydrogen Energy, 43 (27) (2018) 12358–12367. https://doi.org/10.1016/j.ijhydene.2018.04.176
Osinkin DA, Kinetics of CO oxidation and redox cycling of Sr2Fe1.5Mo0.5O6–δ electrode for symmetrical solid state electrochemical devices, J. Power Sources, 418 (2019) 17–23. https://doi.org/10.1016/j.jpowsour.2019.02.026
DOI: https://doi.org/10.15826/elmattech.2023.2.018
Copyright (c) 2023 Denis A. Osinkin

This work is licensed under a Creative Commons Attribution 4.0 International License.

