Viscosity of fluoride melts promising for molten salt nuclear reactors
Abstract
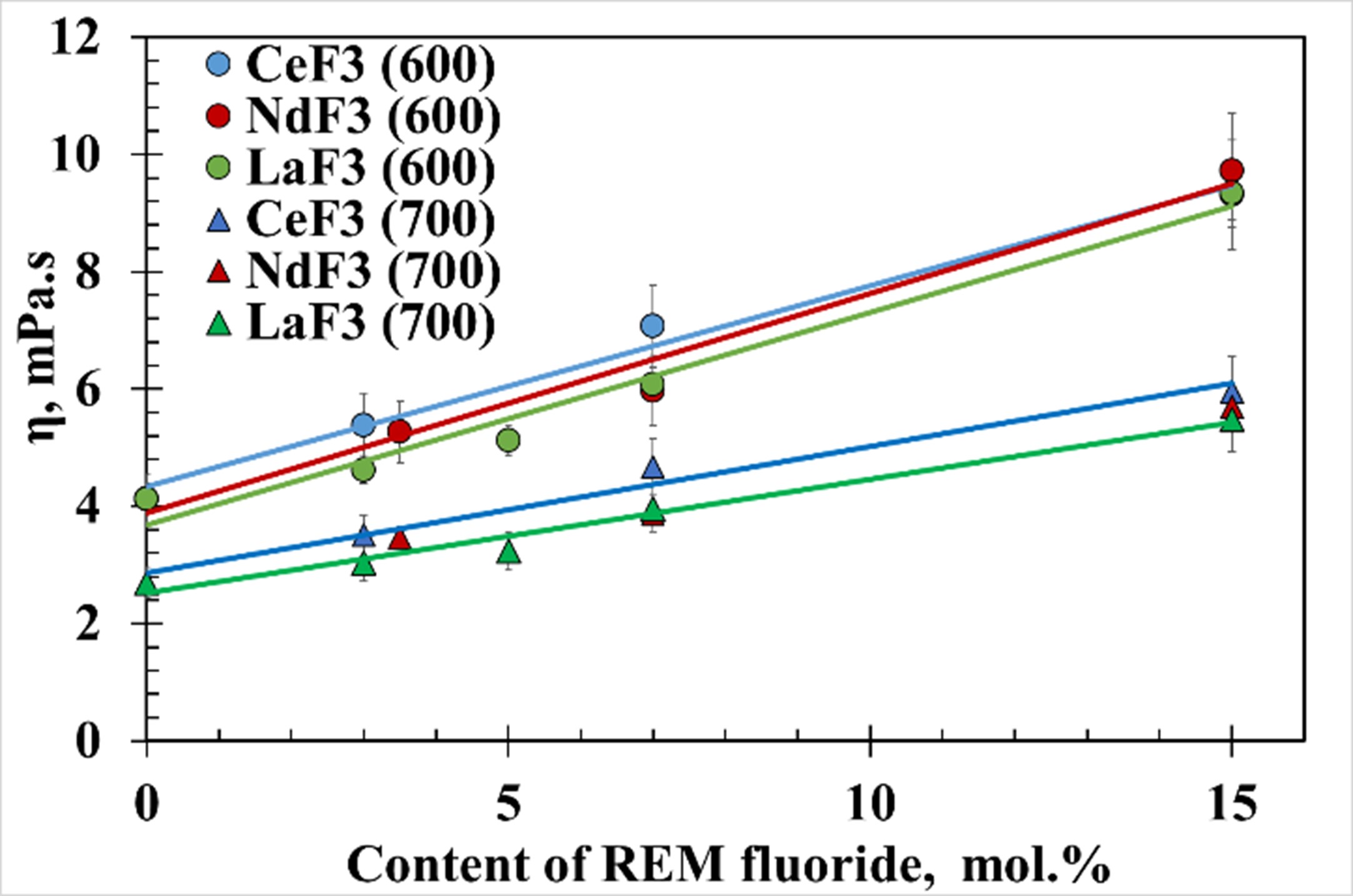
The viscosity of molten salt, as an important hydrodynamic property, should be taken into account when creating and operating molten salt nuclear reactors (MSRs). An eutectic FLiNaK is considered to be one of the most suitable for use in MSR designed for the minor actinides transmutation. The dynamic viscosity of the molten mixtures FLiNaK + NdF3, FLiNaK + CeF3 and FLiNaK + LaF3 was measured in a temperature range of 600–700 °C using the high-temperature rotary rheometer FRS-1600. Lanthanide fluorides were considered as analogues of actinide fluorides. It was revealed that the additions of rare earth fluorides (REM)F3 in amount of 15 mol. % significantly impact the viscosity of the system FLiNaK + (REM)F3,but the effect of NdF3, CeF3 and LaF3 was found to be almost the same. In order to calculate the kinematic viscosity of the molten mixture FLiNaK + NdF3, a regression equation depending on several parameters was derived. This model equation can be used for predicting the kinematic viscosity of molten mixtures of FLiNaK with other rare earth fluorides.
Keywords
Full Text:
PDFReferences
Williams DF, Britt PF. Molten salt chemistry workshop: Report for the US department of energy, office of nuclear energy workshop. Oak Ridge National Laboratory: Oak Ridge, Tennessee, US; 2017. 160 p.
Yu C, Li X, Cai X, Zou C, Analysis of minor actinides transmutation for a Molten Salt Fast Reactor, Annals of Nuclear Energy, 85 (2015) 597–604. https://doi.org/10.1016/j.anucene.2015.06.014
Ashraf O, Tikhomirov GV, Thermal-and fast-spectrum molten salt reactors for minor actinides transmutation, Annals of Nuclear Energy, 148 (2020) 107751. https://doi.org/10.1016/j.anucene.2020.107751
Forsberg CW, Greenspan E. Molten Salt Reactors (MSRs): Coupling Spent Fuel Processing and Actinide Burning. Advances in Nuclear Fuel Management III. American Nuclear Society Hilton Head, South Carolina, US; 2003. 20 p.
He L, Chen L, Xia S, Zou Y, et al., Minor actinides transmutation and 233U breeding in a closed Th-U cycle based on molten chloride salt fast reactor, Energies, 15(24) (2022) 9472–9489. https://doi.org/10.3390/en15249472
Benes O, Konings RJM, Thermodynamic properties and phase diagrams of fluoride salts for nuclear applications, J. Fluorine Chem., 130(1) (2009) 22–29. https://doi.org/10.1016/j.jfluchem.2008.07.014
Magnusson J, Memmott M, Munro T, Review of thermophysical property methods applied to fueled and un-fueled molten salts, Annals of Nuclear Energy, 146 (2020) 107608:1–28. https://doi.org/10.1016/j.anucene.2020.107608
Serrano-Lopez R, Fradera J, Cuesta-Lopez S, Molten salts database for energy applications, Chem Engineering and Processing: Process Intensification, 73 (2013) 87–102. https://doi.org/10.1016/j.cep.2013.07.008
Khokhlov VA, Korzun IA, Dokutovich VN, Filatov ES. Heat capacity and thermal conductivity of molten ternary lithium, sodium, potassium, and zirconium fluorides mixtures, J. of Nuclear Materials, 410(1–3) (2011) 32–38. https://doi.org/10.1016/j.jnucmat.2010.12.306
Khokhlov V A, Ignatiev VV, Afonichkin VK, Evaluating physical properties of molten salt reactor fluoride mixtures, J. of Fluorine Chem., 130(1) (2009) 30–37. https://doi.org/10.1016/j.jfluchem.2008.07.018
Romatoski RR, Hu LW, Fluoride salt coolant properties for nuclear reactor applications: A review, Annals of Nuclear Energy, 109 (2017) 635–647. https://doi.org/10.1016/j.anucene.2017.05.036
Williams DF, Clarno KT, Evaluation of salt coolants for reactor applications, Nucl. Technol., 163(3) (2008) 330–343. https://doi.org/10.13182/NT08-A3992
Britsch K, Anderson M, A critical review of fluoride salt heat transfer, Nuclear Technology, 206(11) (2020) 1625–1641. https://doi.org/10.1080/00295450.2019.1682418
Bahri CNACZ, Al-Areqi WM, Ruf MIFM, Majid AA, Characteristic of molten fluoride salt system LiF-BeF2 (Flibe) and LiF-NaF-KF (Flinak) as coolant and fuel carrier in molten salt reactor (MSR), AIP Conference Proceedings, 1799(1) (2017) 040008:1–8. https://doi.org/10.1063/1.4972932
Lizin AA, Tomilin SV, Gnevashov OE, Gazizov RK, et al., PuF3, AmF3, CeF3, and NdF3 solubility in LiF-NaF-KF melt, At. Energy, 115 (2013) 11–17. https://doi.org/10.1007/s10512-013-9740-9
Seregin MB, Parshin AP, Kuznetsov AYu, Ponomarev LI, Solubility of UF4, ThF4, and CeF3 in a LiF–NaF–KF melt, Radiochemistry, 53(5) (2011) 491–493. https://doi.org/10.1134/S1066362211050079
Ponomarev LI, Seregin MB, Mikhalichenko AA, Parshin AP, et al., Validation of actinide fluoride simulators for studying solubility in fuel salt of molten-salt reactors. At. Energy, 112 (2012) 417–422. https://doi.org/10.1007/s10512-012-9577-7
Mushnikov P, Tkacheva O, Voronin V, Shishkin V, et al., Investigation of the quasi-binary phase diagram FLiNaK-NdF3, Materials, 14 (2021) 6428–6434. https://doi.org/10.3390/ma14216428
Mushnikov P, Tkacheva O, Kholkina A, Zaikov Y, et al., Phase diagram of the quasibinary system LiF-NaF-KF-CeF3, At Energy, 131(5) (2022) 263–267. https://doi.org/10.1007/s10512-022-00876-2
Rudenko AV, Kataev AA, Tkacheva OY, Rotational viscometry for studying the viscosity of cryolite melts, Russian Metallurgy (Metally), 2 (2023) 141–146. https://doi.org/10.1134/S0036029523020192
Rudenko AV, Kataev AA, Tkacheva OY, Dynamic viscosity of the NaF-KF-NdF3 molten system materials, Materials, 15(14) (2022) 4884–4889. https://doi.org/10.3390/ma15144884
Schramm GA. Practical Approach to Rheology and Rheometry; Thermo Electron (Karlsruhe) GmbH: Karlsruhe, Germany, 2004. 259 p.
Tasidou KA, Magnusson J, Munro T, Assael MJ, Reference correlations for the viscosity of molten LiF–NaF–KF, LiF–BeF2, and Li2CO3–Na2CO3–K2CO3, J. Phys. Chem. Ref. Data, 48(4) (2019) 1–9. https://doi.org/10.1063/1.5131349
Cibulková J, Chrenková M, Vasiljev R, Density and viscosity of the (LiF + NaF + KF)eut (1) + K2TaF7 (2) + Ta2O5 (3) melts, J. Chem. Eng. Data, 51(3) (2006) 984–987. https://doi.org/10.1021/je050490g
Merzlyakov A, Ignatiev V, Abalin S, Viscosity of LiF–NaF–KF eutectic and effect of cerium trifluoride and uranium tetra-fluoride additions, Nucl. Eng. Des., 278 (2014) 268–273. https://doi.org/10.1016/j.nucengdes.2014.07.037
Chrenkova M, Danek V, Silny A, Kremenetsky V, et al., Density and viscosity of the (LiF-NaF-KF)eut-KBF4-B2O3 melts, J. Mol. Liq., 102(1–3) (2003) 213–226. https://doi.org/10.1016/S0167-7322(02)00063-6
Kubíková B, Pavlík V, Macková I, Boˇca M, Surface tension and viscosity of the molten (LiF–NaF–KF)eut–K2ZrF6 system, Monatsh. Chem., 143 (2012) 1459–1462. https://doi.org/10.1007/s00706-012-0832-3
Toerklep K, Oeye HA, Viscosity of the eutectic LiF-NaF-KF melt (FLINAK), J. Chem. Eng. Data, 25(1) (1980) 16–20. https://doi.org/10.1021/je60084a007
Tkacheva OYu, Rudenko AV, Kataev AA, Mushnikov PN, et al., The viscosity of molten salts based on the LiF-BeF2 system, Rus J Non-Ferrous Met., 63(3) (2022) 276–283. https://doi.org/10.3103/S1067821222030117
Redkin A, Rudenko A, Khudorozhkova A, Il’ina E, at el., Density and thermal conductivity of some molten mixtures in the FLiNaK-NdF3 system, Submitted in J Chem Thermodynamics.
Galashev AY, Computational Study of the Physical Properties of a High Temperature Molten Salt Mixture of FLiNaK and CeF3, Appl. Sci., 13(2) 2023 1085:1–16. https://doi.org/10.3390/app13021085
Galashev AY, Rakhmanova OR, Abramova KA, Katin KP, et al., Molecular dynamics and experimental study of the effect of CeF3 and NdF3 additives on the physical properties of FLiNaK, J Phys Chem B, 127(5) (2023) 1197–1208. https://doi.org/10.1021/acs.jpcb.2c06915
Zakiryanov D, Fitting the pair potentials for molten salts: A review in brief, Electrochem. Mater. Technol., 2(1) (2023) 20232010:1–9. https://doi.org/10.15826/elmattech.2023.2.010
DOI: https://doi.org/10.15826/elmattech.2023.2.024
Copyright (c) 2023 Olga Yu. Tkacheva, Alexey V. Rudenko, Alexander A. Kataev

This work is licensed under a Creative Commons Attribution 4.0 International License.

