Stability and reproducibility of the amperometric sensors for oxygen concentration analysis in the nitrogen gas mixtures
Abstract
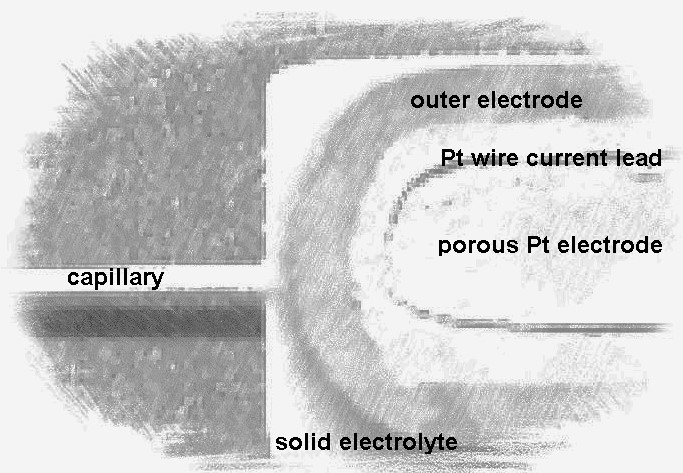
The paper presents experimentally obtained results on the long-term stability tests of the solid electrolyte amperometric sensor output signal during the operation in the O2 + N2 gas mixtures. These data prove the stability and reproducibility of the output signal when measuring the oxygen concentration in air for 8000 hours. The output signal variation during the tests did not exceed ± 2 %. We performed four heating / cooling cycles of different durations, which did not influence either sensor integrity or operation characteristics. The structure of the solid electrolyte sensor and the solid electrolyte/electrode interfacial layer remained unchanged during the tests. Dynamic characteristics of the sensor, including the response time, were stable.
Keywords
Full Text:
PDFReferences
Okamoto H, Obayashi H, Kudo T, Carbon monoxide gas sensor made of stabilized zirconia, Solid State Ion., 1(3–4) (1980) 319–326. https://doi.org/10.1016/0167-2738(80)90012-0
Sorita R, Kawano T, A highly selective CO sensor: screening of electrode materials, Sens. Actuator. B: Chem., 36(1–3) (1996) 274–277. https://doi.org/10.1016/S0925-4005(97)80081-0
Fadeev GI, Kalyakin AS, Somov SI, Electrode potentials of electrochemical cells with oxide-conducting solid electrolyte in chemically nonequilibrium gas mixtures, Russ. J. Electrochem., 45 (2009) 429–433. https://doi.org/10.1134/S1023193509040119
Volkov A, Neimin A, Sosnovsky V, Study of durability of the solid electrolyte electrochemical sensors with etalon electrodes of the Me-MexOy type at measuring of the oxygen concentration of the gaseous media (Issledovaniye dolgovechnosti tverdoelektrolitnykh electrokhimicheskikh datchikov s etalonnymi electrodami tipa Me-MexOy pri izmerenii kislorodsoderzhaniya gazovykh sred), Zavodskaya laboratoriya, 48 (1982) 6–8.
Zhuiykov S, Miura N. Solid-state electrochemical gas sensors for emission control. In: Sorrell CC, Sugihara S, Nowotny J (eds) Materials for energy conversion devices. Cambridge: Woodhead; 2005. pp 303–335.
Park CO, Fergus JW, Miura N, Park J, et al., Solid-state electrochemical gas sensors, Ionics, 15 (2009) 261–284. https://doi.org/10.1007/s11581-008-0300-6
Zhuiykov S. Electrochemistry of zirconia gas sensors. Boca Raton: CRC Press; 2008. pp. 1–297. https://doi.org/10.1201/9781420047622
Shuk P, Bailey E, Guth U, Zirconia Oxygen Sensor for the Process Application: State-of-the-Art, Sens. Transducers, 90 (2008) 174–184. https://doi.org/10.1007/s11581-008-0274-4
Yajma T, Koide K, Fukatsi N, Ohashi T, et al., A new hydrogen sensor for molten aluminum, Sens. Actuators B: Chem., 14(1–3) (1993) 697–699. https://doi.org/10.1016/0925-4005(93)85149-5
Sekhar PK, Brosha EL, Mukundan R, Nelson MA, et al., Development and testing of a miniaturized hydrogen safety sensor prototype, Sens. Actuators B: Chem., 148(2) (2010) 469–477. https://doi.org/10.1016/j.snb.2010.05.031
Miura N, Lu G, Yamazoe N, Progress in mixed-potential type devices based on solid electrolyte for sensing redox gases, Solid State Ion., 136–137 (2000) 533–542. https://doi.org/10.1016/S0167-2738(00)00411-2
Lalauze R, Visconte E, Montanaro L, Pijolat C, A new type of mixed potential sensor using a thick film of beta alumina, Sens. Actuators B: Chem., 13(1–3) (1993) 241–243. https://doi.org/10.1016/0925-4005(93)85371-G
Guillet N, Lalauze R, Pijolat C, Oxygen and carbon monoxide role on the electrical response of a non-Nernstian potentiometric gas sensor; proposition of a model, Sens. Actuators B: Chem., 98(2–3) (2004) 130–139. https://doi.org/10.1016/j.snb.2003.10.001
Morata A, Viricelle JP, Tarancón A, Dezanneau G, et al., Development and characterization of a screen-printed mixed potential gas sensor, Sens. Actuators B: Chem., 130(1) (2008) 561–566. https://doi.org/10.1016/j.snb.2007.09.086
Chevallier L, Di Bartolomeo E, Grilli ML, Mainas M, et al., Non-Nernstian planar sensors based on YSZ with a Nb2O5 electrode, Sens. Actuators B: Chem., 129(2) (2008) 591–598. https://doi.org/10.1016/j.snb.2007.09.037
Park CO, Akbar SA, Weppner W, Ceramic electrolytes and electrochemical sensors, J Mater Sci., 38 (2003) 4639–4660. https://doi.org/10.1023/A:1027454414224
Katahiraa K, Matsumotoa H, Iwaharaa H, Koidea K, et al., A solid electrolyte hydrogen sensor with an electrochemically-supplied hydrogen standard, Sens. Actuators B: Chem., 73(2–3) (2001) 130–134. https://doi.org/10.1016/S0925-4005(00)00672-9
Xia ChY, Lu XCh, Yan Y, Wang T, et al., Improved performances of oxygen potentiometric sensor by electrochemical activation, J Solid State Electrochem., 16 (2012) 2523–2532. https://doi.org/10.1007/s10008-011-1556-8
Pasierb P, Rekas M, Solid-state potentiometric gas sensors – current status and future trends, J Solid State Electrochem., 13 (2009) 3–25. https://doi.org/10.1007/s10008-008-0556-9
Möbius H-H, Hartung R, Solid-state potentiometric gas sensors – a supplement, J Solid State Electrochem., 14 (2010) 669–673. https://doi.org/10.1007/s10008-009-0839-9
Pasierb P, Rekas M, Solid-state potentiometric gas sensors – current status and future trends, J Solid State Electrochem., 13 (2009) 3–25. https://doi.org/10.1007/s10008-008-0556-9
Maskell WC, Steele BCH, Solid state potentiometric oxygen gas sensors, J. Appl. Electrochemistry, 16 (1984) 475–489. https://doi.org/10.1007/BF01006843
Iwahara H, Uchida H, Ogaki K, Nagato H, Nernstian hydrogen sensor using BaCeO3-based, proton-conducting ceramics operative at 200–900 °C, Journal of the Electrochemical Society, 138 (1991) 295–299. https://doi.org/10.1149/1.2085558
Chao Y, Yao S, Buttner WJ, Stetter JR, Amperometric sensor for selective and stable hydrogen measurement, Sens. Actuators B: Chem., 106(2) (2005) 784–790. https://doi.org/10.1016/j.snb.2004.09.042
Lu X, Wu Sh., Wang L., Su Zh., Solid-state amperometric hydrogen sensor based on polymer electrolyte membrane fuel cell, Sens. Actuator B: Chem., 107(2) (2005) 812–817. https://doi.org/10.1016/j.snb.2004.12.022
Tan Y, Tan TC, Sensing behaviour of an amperometric hydrogen sensor, J. Electrochem. Soc., 142 (1995) 1923–1928. https://doi.org/10.1149/1.2044215
Sakthivel M, Weppner W, A portable limiting current solid-state electrochemical diffusion hole type hydrogen sensor device for biomass fuel reactors: engineering aspect, Int. J. Hydrogen Energy, 33(2) (2008) 905–911. https://doi.org/10.1016/j.ijhydene.2007.10.048
Kalyakin AS, Volkov AN, Meshcherskikh AN, Dunyushkina LA, Dual chamber YSZ‑based sensor for simultaneous measurement of methane and water vapor concentrations in CH4 + H2O + N2 gas mixtures, J. Solid State Electrochem., 26 (2022) 739–747. https://doi.org/10.1007/s10008-022-05116-y
Medvedev D, Kalyakin A, Volkov A, Demin A, et al., Electrochemical moisture analysis by combining oxygen- and proton-conducting ceramic electrolytes, Electrochem. commun., 76 (2017) 55–58. https://doi.org/10.1016/j.elecom.2017.01.003
Taniguchi N, Kuroha T, Nishimura C, Iijima K, Characteristics of novel BaZr0.4Ce0.4In0.2O3 proton conducting ceramics and their application to hydrogen sensors, Solid State Ion., 176(39–40) (2005) 2979–2983. https://doi.org/10.1016/j.ssi.2005.09.035
Tan Y, Tan TC, Sensing behaviour of an amperometric hydrogen sensor, J. Electrochem. Soc., 142(6) (1995) 1923–1928. https://doi.org/10.1149/1.2044215
Bao J, Okuyama Y, Shi Z, Ohno H, et al., Properties of Electrical Conductivity in Y-Doped CaZrO3, Mater. Trans., 53(5) (2012) 973-979. https://doi.org/10.2320/matertrans.m2012017
Goppel W, Reinhardt G, Rasch M, Trends in development of solid state ampermetric and potentiometric high temperature sensors, Solid State Ion., 136–137 (2000) 519–531. https://doi.org/10.1016/S0167-2738(00)00410-0
Dubbe A, Fundamentals of solid state ionic micro gas sensors, Sens. Actuators B: Chem., 88(2) (2003) 138–148. https://doi.org/10.1016/S0925-4005(02)00317-9
Somov SI, Reinhardt G, Guth U, Göpel W, Tubular amperometric high-temperature sensors: simultaneous determination of oxygen, nitrogen oxides and combustible components, Sens. Actuators B: Chem., 65(1–3) (2000) 68–69. https://doi.org/10.1016/S0925-4005(99)00341-X
Usui T, Asada A, Nakazawa M, Osanai H, Gas Polarographic oxygen sensor using an oxygen/Zirconia electrolyte, J. Electrochem. Soc., 136 (1989) 534–542. https://doi.org/10.1149/1.2096676
DOI: https://doi.org/10.15826/elmattech.2024.3.027
Copyright (c) 2024 Aleksander N. Volkov, Anatoly S. Kalyakin, Daria P. Mirzayants

This work is licensed under a Creative Commons Attribution 4.0 International License.

