A comprehensive study of the thermal behavior of rare earth chloride hydrates: resolving contradictions
Abstract
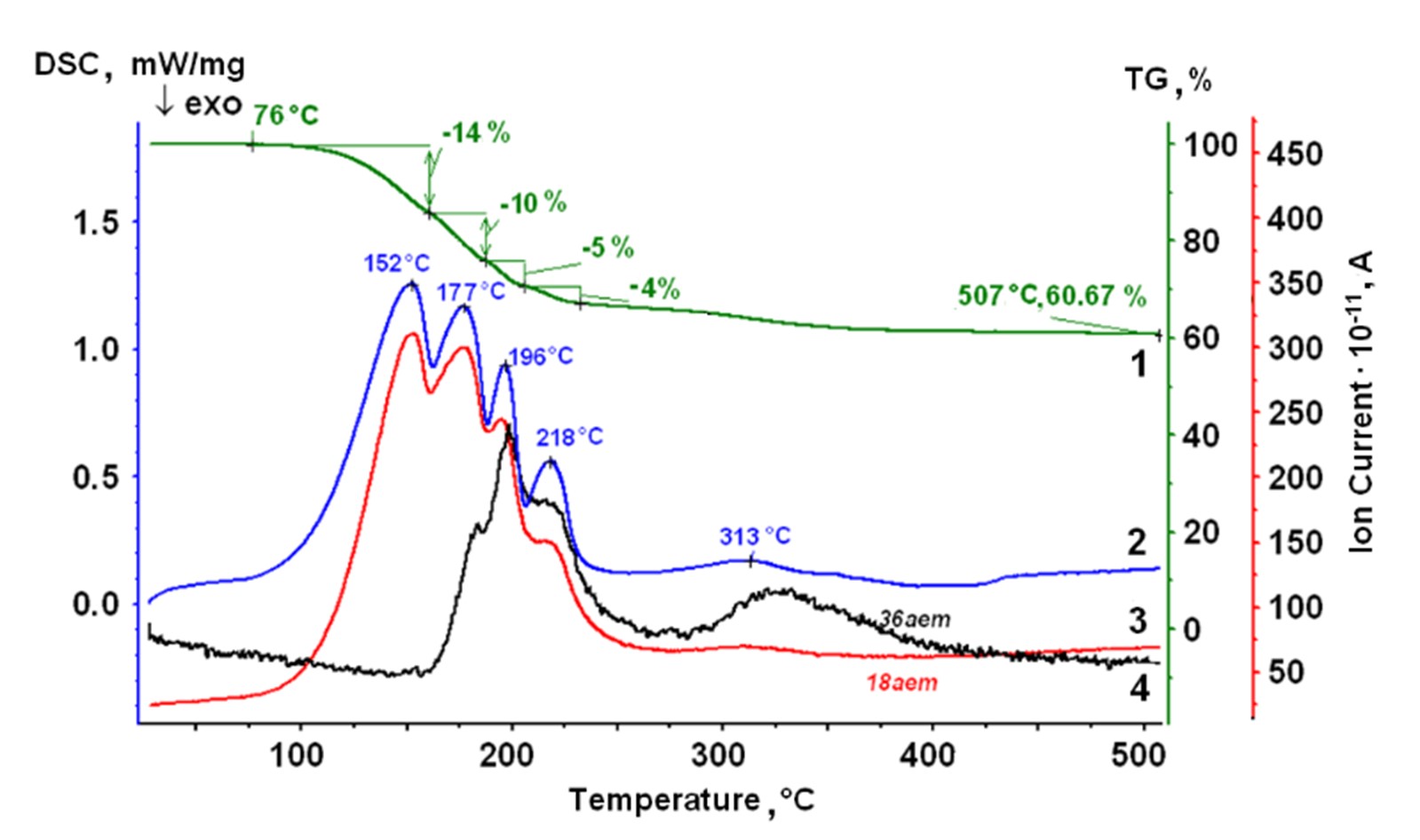
Rare earth metal chlorides are used as starting materials on electrochemical synthesis of pure metals. These chlorides are hygroscopic and tend to form hydrates. To resolve the uncertainty of the thermal behavior of the hydrates LaCl3 ∙ 7H2O, NdCl3 ∙ 6H2O, SmCl3 ∙ 6H2O, and YbCl3 ∙ 6H2O, a comprehensive study is carried out. Thermogravimetry, differential scanning calorimetry and mass spectrometry were used to reveal the dehydration and hydrolysis processes over the temperature range of 25–700 ºC. Phase compositions of the initial hydrates, intermediate and final products are investigated by the XRD analysis, Raman and IR spectroscopy. Distinctions in the thermal behavior of the studied hydrates were found. The features must be taken into account when obtaining anhydrous rare earth chlorides and in technological processes with its participation.
Keywords
Full Text:
PDFReferences
Zhang QY, Huang XY, Recent progress in quantum cutting phosphors, Progress Mat. Sci., 55(5) (2010) 353–427. https://doi.org/10.1016/j.pmatsci.2009.10.001
Xu D, Zhang Y, Zhang D, Yang S, Structural, luminescence and magnetic properties of Yb3+-Er3+ codoped Gd2O3 hierarchical architectures, Eng. Comm., 17(5) (2015) 1106–1114. https://doi.org/10.1039/C4CE01970A
Gupta CK, Krishnamurthy N. Extractive Metallurgy of Rare Earths. London. New York. Washington: CRC PRESS; 2005. 522 p.
Kodina GE, Kulakov VN, Sheino IN, Rare earth elements in nuclear medicine (review). Saratov J. Med. Sci. Res. [Internet], 10(4) (2014) 849–858. Russian. Available from: https://www.ssmj.ru/system/files/2014_04-01_849-858.pdf
Ji N, Zhu T, Peng H, Jiang F, et al., The Electrolytic Reduction of Gd2O3 in LiCl-KCl-Li2O Molten Salt, Electrochem. Soc., 168 (2021) 082512. https://doi.org/10.1149/1945-7111/ac1f59
Liu K, Liu Y-L, Yuan L-Y, Zhao X-L, et al. Electroextraction of gadolinium from Gd2O3 in LiCl–KCl–AlCl3 molten salts, Electrochim. Acta, 109 (2013) 732–740. https://doi.org/10.1016/j.electacta.2013.07.084
Guo X. Dissolution and Electrochemical Reduction of Rare Earth Oxides in Fluoride Electrolytes (dissertation) (TU Delft), Delft University of Technology; 2021. 188 p. https://doi.org/10.4233/uuid:79a1f6f1-52d1-48a9-a0df-03c1b1ce0ac6
Wendlandt W, The thermal decomposition of yttrium, scandium, and some rare-earth chloride hydrates, J. Inorg. Nucl. Chem., 5(2) (1957) 118–122. https://doi.org/10.1016/0022-1902(57)80052-9
Wendlandt W. The thermal decomposition of the heavier rare earth metal chloride hydrates, J. Inorg. Nucl. Chem., 9(2) (1959) 136–139. https://doi.org/10.1016/0022-1902(59)80072-5
Korzun IV, Zakir’yanova ID, Nikolaeva EV, Mechanism and Caloric Effects of the Thermal Dehydration of GdCl3 ⋅ 6H2O Crystalline Hydrate, Russian Metallurgy (Metally), 2018 (2018) 722–727. https://doi.org/10.1134/S0036029518080104
Zakiryanova ID, Zakiryanov DO, Zakiryanov PO, Local structure and dynamics of ions in LiCl-GdCl3, KCl-GdCl3 and LiCl-GdCl3-Gd2O3 melts: Ab initio molecular dynamics simulations and Raman spectroscopy, J. Mol. Liq., 376 (2023), 121485. https://doi.org/10.1016/j.molliq.2023.121485
Nikolaeva EV, Zakiryanova ID, Bovet AL, Korzun IV, Electrical conductivity of GdCl3–LiCl and GdCl3–LiCl-Gd2O3 molten systems, J. Serb. Chem. Soc., 88(11) (2023) 1135–1147. https://doi.org/10.2298/JSC230131051N
Zakiryanov D, The impact of oxide impurity on the structure of FLiNaK and FLiNaK–CeF3 melts: A simulation study, J. Mol. Liq., 384 (2023) 122265. https://doi.org/10.1016/j.molliq.2023.122265
Zakiryanov DO, Zakiryanova ID, Tkachev NK, Study of local structure and ion dynamics in GdCl3-Gd2O3 and KCl-GdCl3-Gd2O3 melts: In situ Raman spectroscopy and ab initio molecular dynamics, J. Mol. Liq., 301 (2020) 112396 https://doi.org/10.1016/j.molliq.2019.112396
Korzun IV, Nikolaeva EV, Zakir’yanova ID, Thermal analysis of the oxide–chloride systems GdCl3–Gd2O3 and GdCl3–KCl–Gd2O3, J. Thermoanal. Calorim., 144 (2020) 1343–1349. https://doi.org/10.1007/s10973-020-09558-2
Ashcroft SJ, Mortimer CT, The thermal decomposition of lanthanide (III) chloride hydrates, J. Less-Com. Met., 14(4) (1968) 403–406. https://doi.org/10.1016/0022-5088(68)90164-1
Sokolova NP, Ukraintseva EA, Dissociation pressure of some crystalline hydrates of lanthanide chlorides, Izv. Siberian Branch of the USSR Acad, Sci. Ser. chem. sci., 3 (1984) 24–26. Russian.
Ukraintseva EL, Sokolova NP, Logvinenko VA, Thermodynamic characteristics of dehydration of hepta- and hexahydrates of lanthanide chlorides of the cerium subgroup. Radiochem., 29 (1987) 481–485. Russian.
Ukraintseva EA, Sokolova NP, Logvinenko VA, Thermodynamic characteristics of dehydration of erbium, thulium and lutetium chloride hexahydrates. Radiochem., 31 (1991) 78–80. Russian.
Haeseler G, Matthes F, Uber den thermischen Abbau dek Chloridhydrate der Elemente der seltenen erden, J. Less-Comm. Met., 9(2) (1965) I33–151. https://doi.org/10.1016/0022-5088(65)90090-1
Hong VV, Sundstrum J, The dehydration schemes of rare-earth chlorides, Thermochim. Acta., 307(1) (1997) 37–43. https://doi.org/10.1016/S0040-6031(97)00296-7
Lee T, Cho Y, Eun H, Son S, et al. A study on dehydration of rare earth chloride hydrate, J. Korean Radioactive Waste Soc., 10(2) (2012) 125–132. https://doi.org/10.7733/JKRWS.2012.10.2.125
Kipouros GJ, Sharma RA, Characterization of neodymium trichloride hydrates and neodymium hydroxychloride, J. Less-Com, Met., 160(1) (1990) 85–99. https://doi.org/10.1016/0022-5088(90)90110-6
Sundstrum J, Hong VV, Investigation of the kinetics of the fluidized bed process for the dehydration of NdCl3 · 6H2O, TbCl3 · 6H2O and DyCl3 · 6H2O. Thermochim. Acta., 306(1–2) (1997) 13–21. https://doi.org/10.1016/S0040-6031(97)00293-1
Sahoo DK, Thakur S, Mishra R, Determination of thermodynamic stability of neodymium chloride hydrates (NdCl3 · xH2O) by dynamic transpiration method, J. Therm. Anal. Calorim., 126 (2016) 1407–1415. https://doi.org/10.1007/s10973-016-5714-1
Grasselli JG, Snavely MK, Balkin BJ. Chemical applications of Raman Spectroscopy. N. York. Toronto: Wiley Publ.; 1973. 216 p.
Kochedykov VA, Zakiryanova ID, Akashev LA, Identification of products of interaction of oxides of rare earth metals with components of the air atmosphere using IR spectroscopy, Analytics and control, 10 (2006) 172–174. Russian. URI: http://elar.urfu.ru/handle/10995/58134
Kochedykov VA, Zakiryanova ID, Korzun IV, Study of the thermal decomposition of the products of interaction of rare earth oxides with components of the air atmosphere, Analytics and control, 9 (2005) 58–63. Russian. URI: http://elar.urfu.ru/handle/10995/58914
Makatun VN. Chemistry of inorganic hydrates. Moscow: Science and Technology; 1985. 245 p. Russian.
Hase Y, Dunstan POL, Temperini MLA, Raman active normal vibrations of lanthanide oxychlorides, Spectrochim. Acta. Part A: Mol. Spectroscopy, 37(8) (1981) 597–599. https://doi.org/10.1016/0584-8539(81)80055-4
Basile LJ, Ferraro JR, Gronert D, I.R. Spectra of several lanthanide oxyhalides, J. Inorg. Nuclear Chem., 33(4) (1971) 1074–1053. https://doi.org/10.1016/0022-1902(71)80173-2
Zakiryanova ID, In situ Raman Spectroscopic Study of the Kinetics of the Chemical Dissolution of Ytterbium Oxide in YbCl3–KCl Melt, J. Appl. Spec., 88 (2021) 755–760. https://doi.org/10.1007/s10812-021-01236-x
Zakiryanova ID, Zakiryanov DO, Ab initio molecular dynamics simulations and Raman spectra of the YbCl3 - KCl and Yb2O3 - YbCl3 - KCl ionic melts, J. Mol, Liq, 318 (2020) 114054. https://doi.org/10.1016/j.molliq.2020.114054
Revzin GE, Anhydrous chlorides of rare earth elements and scandium. In: Methods of Obtaining of Chemical Reagents and Preparations. Moscow: Science and Technology; 1967. p. 16–27. Russian.
DOI: https://doi.org/10.15826/elmattech.2024.3.028
Copyright (c) 2024 Irina D. Zakiryanova, Iraida V. Korzun, Emma G. Vovkotrub, Boris D. Antonov

This work is licensed under a Creative Commons Attribution 4.0 International License.

