Current state of research on the viscosity of molten fluorides
Abstract
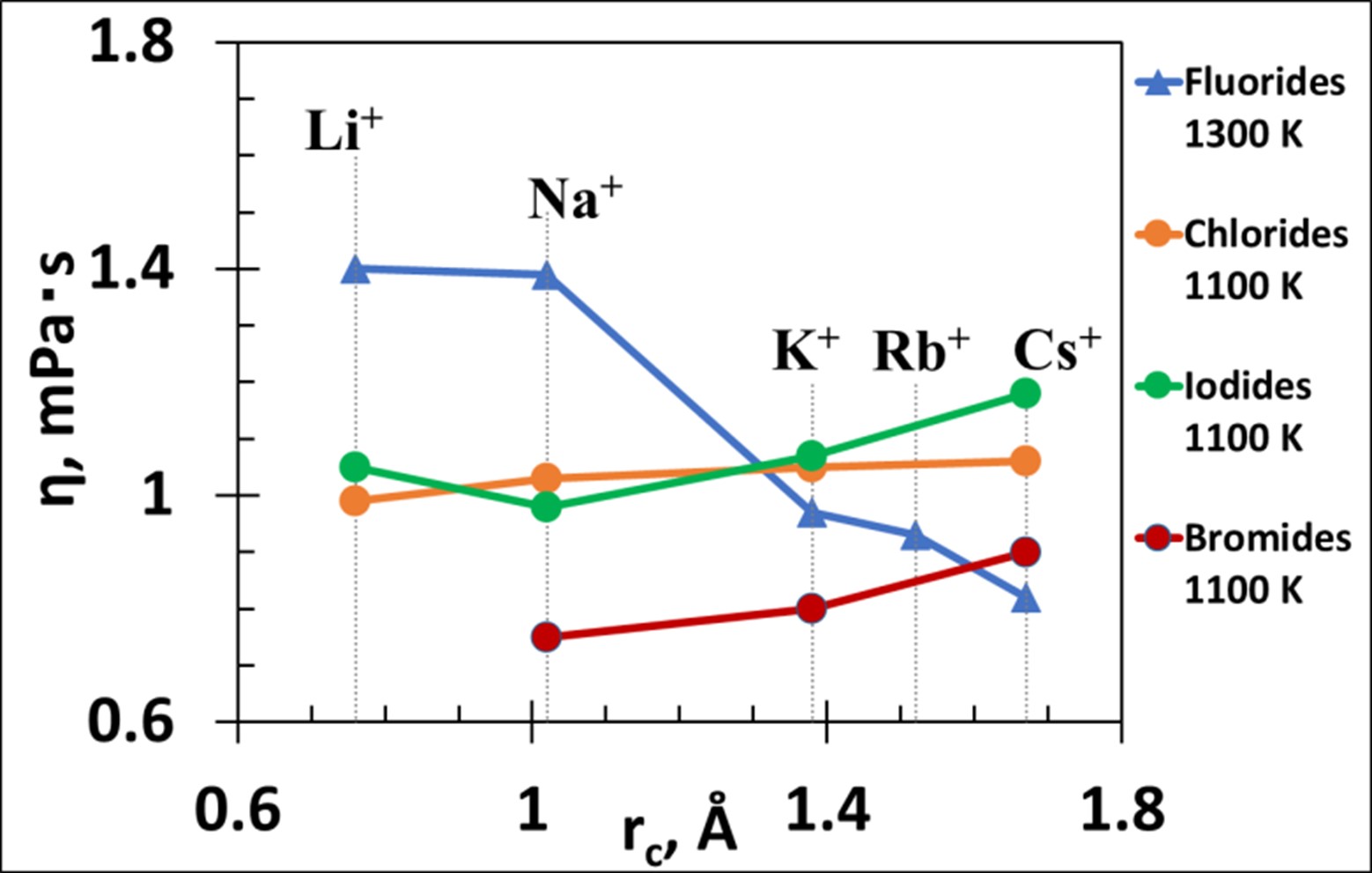
The development of new high-precision equipment for the experimental study of the physical-chemical properties of molten salts has led to the emergence of new data. This review examines recent experimental results on the viscosity of molten salt mixtures based on alkali fluorides, which are currently used in modern technologies for the production of metals and alloys and are also promising media for application in molten salt nuclear reactors. Various approaches to the analysis of experimental data are discussed. A different mechanism of viscous flow in the series LiF – NaF – KF and KF – RbF – CsF is well explained from the position of the theory of the autocomplex structure of molten salts. An anomalous decrease in the viscosity of fluorides from lithium to cesium, in comparison with other alkali metal halides, the viscosity of which increases with increasing the cation radius, is confirmed by calculations of the formation energy of the autocomplex and the binding energy between the complex-forming ion and the free ion of the second coordination sphere. To study the mechanism of liquid flow, the temperature dependence of dynamic viscosity is analyzed using the fluidity parameter. Additional information regarding the mechanism of viscous flow can be obtained from the relationship between the viscosity and electrical conductivity of melts. It was assumed that the most promising approach to studying the kinematic properties and the structure of molten salts is the use of both high-precision experiment and mathematical modeling based on first principles.
Keywords
Full Text:
PDFReferences
Suzdaltsev AV, Pershin PS, Filatov AA, Nikolaev AYu, et al., Synthesis of aluminum master alloys in oxide-fluoride melts: a Review, J. Electrochem. Soc., 167(10) (2020) 102503. https://doi.org/10.1149/1945-7111/ab9879
Barnes J, Coutts R, Horne T, Thai J, Characterization of molten salts for application in molten salt reactors, PAM Review Energy Science & Technology, 6 (2019) 68412. https://doi.org/10.5130/pamr.v6i0.1546
Britsch K, Anderson M, A critical review of fluoride salt heat transfer, Nuclear Techn., 206(11) (2020) 1625–1641. https://doi.org/10.1080/00295450.2019.1682418
Ponomarev LI, Seregin MB, Parshin AP, Mel’nikov SA, et al., Fuel salt for the molten-salt reactor, Atomic Energy, 115(1) (2013) 5–10. https://doi.org/10.1007/s10512-013-9739-2
Kuchibhotla A, Banerjee D, Dhir V, Forced convection heat transfer of molten salts: A review, Nuclear Engineering and Design, 362(3) (2020) 110591. https://doi.org/10.1016/j.nucengdes.2020.110591
Magnusson J, Memmott M, Munro T, Review of thermophysical property methods applied to fueled and un-fueled molten salts, Annals of Nuclear Energy, 146 (2020) 107608. https://doi.org/10.1016/j.anucene.2020.107608
Tasidou KA, Chliatzou ChD, Assael MJ, Antoniadis KD, et al., Reference correlations for the viscosity of 13 inorganic molten salts, J. Phys. Chem. Ref. Data, 48(1) (2019) 013101. https://doi.org/10.1063/1.5091511
Janz GJ, Allen CB, Bansal NP, Murphy RM, et al. Physical properties data compilations relevant to energy storage. II. Molten salts: data on single and multi-component salt systems molten salts data center. New York: Cogswell Laboratory Rensselaer Polytechnic Institute Troy; 1979. 435 p.
Brockner W, Grjotheim K, Ohta T, Øye HA, High-temperature viscometer for fluid liquids. Part II. Viscosities of the alkali chlorides, Ber. Bunsenges. Phys. Chem., 79(4) (1975) 344–347. https://doi.org/10.1002/BBPC.19750790406
Ejima T, Shimakage K, Sato Y, Okuda H, et al., Viscosity measurement of alkali chlorides with capillary viscometer, Nippon Kagaku Kaishi, 6 (1982) 961–968.
Murgulescu IG, Zuca S, Internal friction of molten salts. J. Phys. Chem. (Leipzig), 222 (1963) 300–310.
Janz GJ, Thermodynamic and transport properties for molten salts: correlation equations for critically evaluated density, surface tension, electrical conductance, and viscosity data, Phys. Chem. Ref. Data, 17 (Suppl. No. 2) (1988) 1–309.
Chen Y, Wu Y, Ren N, Ma C, Experimental study of viscosity characteristics of high temperature heat transfer molten salts, Sci. China Tech. Sci., 54 (2011) 3022–3026. https://doi.org/10.1007/s11431-011-4530-x
Kubíková B, Pavlík V, Macková I, Boˇca M, Surface tension and viscosity of the molten (LiF–NaF–KF)eut–K2ZrF6 system, Monatsh. Chem., 143 (2012) 1459–1462. https://doi.org/10.1007/s00706-012-0832-3
Cibulková J, Chrenková M, Vasiljev R, Kremenetsky V, et al., Density and viscosity of the (LiF + NaF + KF)eut (1) + K2TaF7 (2) + Ta2O5 (3) melts, J. Chem. Eng. Data, 51(3) (2006) 984–987. https://doi.org/10.1021/je050490g
Chrenkova M, Danek V, Silny A, Kremenetsky V, et al., Density and viscosity of the (LiF-NaF-KF)eut-KBF4-B2O3 melts, J. Mol. Liq., 102(1–3) (2003) 213–226. https://doi.org/10.1016/S0167-7322(02)00063-6
Merzlyakov A, Ignatiev V, Abalin S, Viscosity of LiF–NaF–KF eutectic and effect of cerium trifluoride and uranium tetra-fluoride additions, Nucl. Eng. Des., 278 (2014) 268–273. https://doi.org/10.1016/j.nucengdes.2014.07.037
Popescu A-M, Constantin V, Viscosity of alkali fluoride ionic melts at temperatures up to 373.15 K above melting points, Chem. Eng. Comm., 202(12) (2015) 1703–1710. http://dx.doi.org/10.1080/00986445.2014.970254
An X, Cheng J, Su T, Determination of thermal physical properties of alkali fluoride/carbonate eutectic molten salt, AIP Conf. Proc., 1850(1) (2017) 070001. https://doi.org/10.1063/1.4984415
Rudenko AV, Kataev AA, Tkacheva OYu, Rotational viscometry for studying the viscosity of cryolite melts, Russian Metallurgy (Metally), 2 2023 141–146. https://doi.org/10.1134/S0036029523020192
Merzlyakov AV, Ignatiev VV, Abalin SS, Viscosity of molten lithium, thorium and beryllium fluorides mixtures, J. Nucl. Mat., 419(1–3) (2011) 361–365. https://doi.org/10.1016/j.jnucmat.2011.06.030
Abe Y, Kosugiyama O, Nagashima A, Viscosity of LiF–BeF2 eutectic mixture (xBeF2 = 0.328) and LiF single salt at elevated temperatures, J. Nucl. Mater., 99(2–3) (1981) 173–183. https://doi.org/10.1016/0022-3115(81)90186-0
Rudenko AV, Kataev AA, Tkacheva OYu, Zaikov YuP, et al., Viscosity of conventional cryolite-alumina melts, Russ. J. Non-ferrous Metals, 63(1) (2022) 1–6. https://doi.org/10.3103/S1067821222010102
Schramm GA. Practical approach to rheology and rheometry. Karlsruhe: Gebrueder HAAKE; 1994. 290 p.
Tkacheva OYu, Rudenko AV, Kataev AA, Viscosity of fluoride melts promising for molten salt nuclear reactors, Electrochem. Mater. Technol., 2(4) (2023) 20232024. https://doi.org/10.15826/elmattech.2023.2.024
Tkacheva OYu, Rudenko AV, Kataev AA, Mushnikov PN, et al., The viscosity of molten salts based on the LiF–BeF2 system, Metallurgy Nonferr. Metals, 63(3) (2022) 276–283. https://doi.org/10.3103/S1067821222030117
Cohen SI, Jones TN. Viscosity measurements on molten fluoride mixtures. Report No. ORNL-2278. Oak Ridge, TN, USA: Oak Ridge National Laboratory; 1957.
Shabanov OM, Gadzhiev SM. Intensification of ion transport in molten electrolytes by stimulated dissociation of complex ions. India, UK: BP International; 2021. 84 p.
Minchenko VI, Stepanov VP. Ionic melts. elastic and caloric properties. Yekaterinburg, Russia: Ural Branch of the Russian Academy of Sciences; 2008. 367 p. (Russian)
Shannon RD, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., A32 (1976) 751−767.
Sugiura Y, Saito Y, Endo T, Makita Y, Effect of the ionic radius of alkali metal ions on octacalcium phosphate formation via different substitution modes, Cryst. Growth Des., 19(7) (2019) 4162−4171. https://doi.org/10.1021/acs.cgd.9b00656
Nguyen DK, Danek V, Viscosity of melts of the system LiF–KF–K2NbF7, Chem. Papers, 54(5) (2000) 277–281.
Rudenko AV, Kataev AA, Tkacheva OYu, Dynamic viscosity of the NaF-KF-NdF3 molten system materials, Materials, 15(14) (2022) 4884–4889. https://doi.org/10.3390/ma15144884
Tasidou KA, Magnusson J, Munro T, Assael MJ, Reference correlations for the viscosity of molten LiF–NaF–KF, LiF–BeF2, and Li2CO3–Na2CO3–K2CO3, J. Phys. Chem. Ref. Data, 48(4) (2019) 1–9. https://doi.org/10.1063/1.5131349
Janz GJ, Tomkins RPT. Physical Properties Data Compilations Relevant to Energy Storage. IV. Molten salts: Data on Additional Single and Multi-Component Salt Systems, NSRDS-NBS 61, Part IV, Molten Salts Data Center. Troy, NY, USA: Cogswell Laboratory. Rensselaer Polytechnic Institute; 1981.
Tørklep K, Øye HA, Viscosity of the eutectic LiF-NaF-KF melt (FLINAK), J. Chem. Eng. Data, 25 (1980) 16–20. https://doi.org/10.1021/je60084a007
Williams DF, Clarno KT, Evaluation of salt coolants for reactor applications, Nucl. Technol., 163(3) (2008) 330–343. https://doi.org/10.13182/NT08-A3992
Janz GJ, Tomkins RPT, Allen CB, Gardner GL, Molten salts: Volume 4, Part 2, Chlorides and mixtures - electrical conductance, density, viscosity, and surface tension data, J. Phys. Chem. Reference Data, 4 (1975) 871–1015. https://doi.org/10.1063/1.555527
Cantor S, Cooke JW, Dworkin AS, Robbins GD, et al. Physical properties of molten-salt reactor fuel, coolant, and flush salts: report. NY, USA: Oak Ridge National Laboratory; 1968. 55 p. No. ORNL-TM-2316
Merzlyakov AV, Ignat’ev VV, Abalin SS, Measurement of the kinematic viscosity of molar melt 73LiF–27BeF2 and influence of cerium trifluoride and zirconium tetrafluoride additives on viscosity, Atomic Energy, 125(2) (2018) 91–94. https://doi.org/10.1007/s10512-018-0447-9
Robelin C, Chartrand C, A viscosity model for the (NaF + AlF3 + CaF2 + Al2O3) electrolyte, J. Chem. Thermodynamics, 43(5) (2011) 764–774. https://doi.org/10.1016/j.jct.2010.12.017
Tkacheva O, Arkhipov P, Kataev A, Rudenko A, et al., Electrolyte viscosity and solid phase formation during aluminium electrolysis, Electrochem. Commun., 122 (2021) 106893. https://doi.org/10.1016/j.elecom.2020.106893
Lyutina AS, Kataev AA, Rudenko AV, Tkacheva OYu, Effect of Al2O3 and CaF2 additives on the viscosity of conventional cryolite melts, Chem. Tech. Acta, 8(3) 2021 20218306 https://doi.org/10.15826/chimtech.2021.8.3.06
Korenko M, Vaskova Z, Priscak J, Density, viscosity and electrical conductivity of the molten cryolite electrolytes (Na3AlF6–SiO2) for solar grade silicon (Si-SoG) electrowinning, Silicon, 7 (2015) 261–267. https://doi.org/10.1007/s12633-014-9214-2
Torklep K, Oye H, Viscosity of NaF–AlF3–Al2O3 melt mixtures, Electrochimica Acta, 25(2) (1980) 229–235. https://doi.org/10.1016/0013-4686(80)80048-X
Silný A, Chrenková M, Daněk V, Vasiljev R, et al., Density, viscosity, surface tension, and interfacial tension in the systems NaF(KF) + AlF3, J. Chem. Eng. Data, 49(6) (2004) 1542–1545 https://doi.org/10.1021/je0341965
Volarovich MP, Application of the formula of A.I. Bachinsky for the viscosity of molten salts at high temperatures, Proceedings of the USSR Academy of Sciences. VII series. Department of Mathematical and Natural Sciences, 10 (1933) 1431–1437 (Russian).
Ertl H, Dullien FAL, Hildebrand's equations for viscosity and diffusivity, J. Phys. Chem., 77(25) (1973) 3007–3011. https://doi.org/10.1021/j100643a016
Marcus Y, The fluidity of molten salts re-examined, Fluid Phase Equilibria, 366 (2014) 57–60. https://doi.org/10.1016/j.fluid.2014.01.011
Chhabra RP, Hunter RJ, The fluidity of molten salts, Rheol. Acta, 20 (1981) 203–206. https://doi.org/10.1007/BF01513063
Chhabra RP, Shridhar T, Estimation of viscosity of liquid mixtures using Hildebrand’s fluidity model, Chem. Eng. data, 40(1) (1989) 39–43. https://doi.org/10.1016/0300-9467(89)80042-5
Veliyulin E, Voronel A, Oye HA, Universal features in the viscosity behaviour of salt melts and their mixtures, J. Phys.: Condens. Matter, 7 (1995) 4821–4828. https://doi.org/10.1088/0953-8984/7/25/007
Salanne M, Simon C, Turq P, Madden PA, Conductivity-viscosity-structure: unpicking the relationship in an ionic liquid, J. Phys. Chem. B, 111(18) (2007) 4678–4684. https://doi.org/10.1021/jp067073a
Dai J, Han H, Li O, Huai P, First-principle investigation of the structure and vibrational spectra of the local structures in LiF–BeF2 Molten Salts, J. Mol. Liq. 213 (2016) 17–22. http://dx.doi.org/10.1016/j.molliq.2015.10.053
Frenkel J. Kinetic theory of liquids. Mineola, NY, USA: Dover Publications; 1955. 488 p.
Arkhipov P, Tkacheva O, The electrical conductivity of molten oxide-fluoride cryolite mixtures, Materials, 14(23) (2021) 7419. https://doi.org/10.3390/ma14237419
Galashev AY, Rakhmanova OR, Abramova KA, Katin KP, et al., Molecular dynamics and experimental study of the effect of CeF3 and NdF3 additives on the physical properties of FLiNaK, J. Phys. Chem. B, 127(5) (2023) 1197–1208. https://doi.org/10.1021/acs.jpcb.2c06915
Zakiryanov DO, Fitting the pair potentials for molten salts: A review in brief, Electrochem. Mater. Technol., 2(1) (2023) 20232010. https://doi.org/10.15826/elmattech.2023.2.010
Zakiryanov DO, Applying the Born-Mayer model to describe the physicochemical properties of FLiNaK ternary melt, Computational and Theoretical Chemistry, 1219 (2023) 113951. https://doi.org/10.1016/j.comptc.2022.113951
Rodriguez A, Lam S, Hu M. Thermodynamic and transport properties of LiF and FLiBe molten salts with deep learning potentials//ACS Appl. Interfaces, 13(46) (2021) 55367–55379. https://doi.org/10.1021/acsami.1c17942
DOI: https://doi.org/10.15826/elmattech.2024.3.029
Copyright (c) 2024 Olga Yu. Tkacheva

This work is licensed under a Creative Commons Attribution 4.0 International License.

