Structure and properties of the FLiNaK – LaF3 melt obtained with neural network potential
Abstract
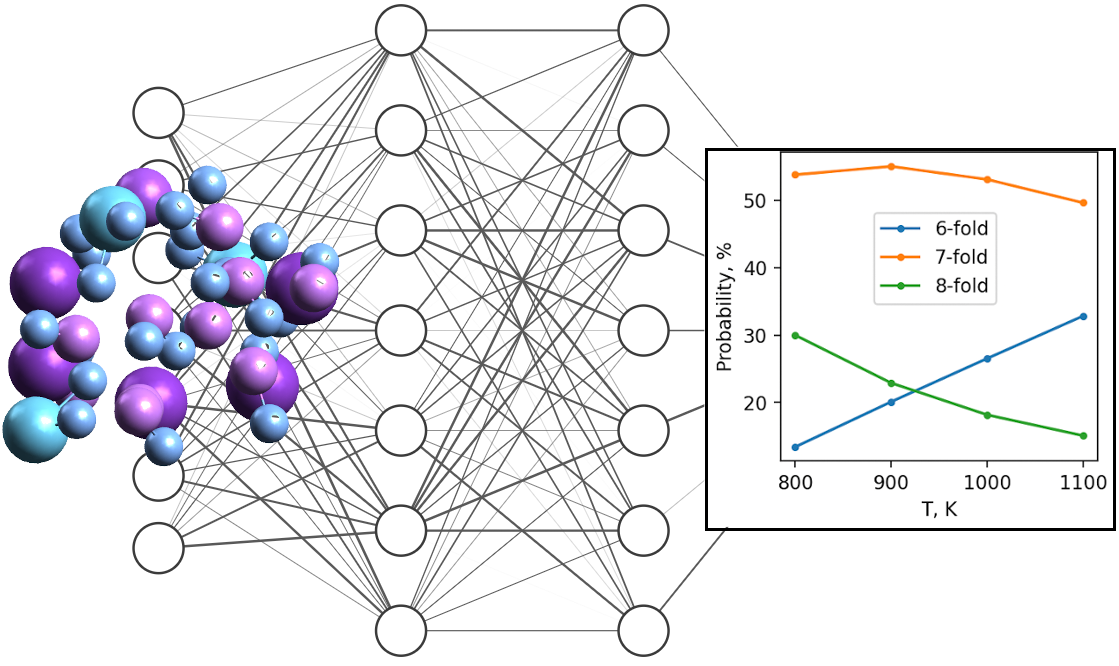
Accurate and efficient prediction of the thermochemical properties of the melts applicable in molten salt reactors could be made if the proper machine learning model is used. In this paper, the neural network potential for simulation of 85 % (LiF – NaF – KF)eut. – 15 % LaF3 molten mixture was developed based on ab initio data. In spite of multiple atomic types, the model of a moderate size showed small root mean squared errors in energy and forces of 0.5 meV/atom and 39 meV/Å, respectively. Then the neural network potential was employed to calculate local structure, density, self-diffusion coefficients, heat capacity, thermal conductivity, and thermal diffusivity for a range of temperatures. We found that the addition of LaF3 to the eutectic mixture of alkali fluorides results in a reduction the melt ability to store and transfer heat. The strong effect observed here is the reduction in heat capacity by 20–30 %. The analysis of the local structure details reveals the existence of [LaF6], [LaF7] and [LaF8] groupings, with the most probable being [LaF7] and the La – F separation averaged over the ensemble of 2.35 Å.
Keywords
Full Text:
PDFReferences
Ignatiev VV, Feynberg OS, Zagnitko AV, Merzlyakov AV, et al., Molten-salt reactors: new possibilities, problems and solutions, Atomic Energy, 112 (2012) 157–165. https://doi.org/10.1007/s10512-012-9537-2
LeBlanc D, Molten salt reactors: A new beginning for an old idea, Nuclear Engineering and Design, 240(6) (2010) 1644–1656. https://doi.org/10.1016/j.nucengdes.2009.12.033
Serp J, Allibert M, Beneš O, Delpech S, et al., The molten salt reactor (MSR) in generation IV: Overview and perspectives, Progress in Nuclear Energy, 77 (2014) 308–319. https://doi.org/10.1016/j.pnucene.2014.02.014
Roper R, Harkema M, Sabharwall P, Riddle C, et al., Molten salt for advanced energy applications: A review, Annals of Nuclear Energy, 169 (2022) 108924. https://doi.org/10.1016/j.anucene.2021.108924
Ponomarev LI, Seregin MB, Mihalichenko AA, Parshin AP, et al., Substantiation of the choice of actinide fluoride imitators for the study of solubility in the fuel salt of molten-salt reactors (in Russian), Atom. Energiya, 112 (2012).
Ackerman JP, Chemical basis for pyrochemical reprocessing of nuclear fuel, Industrial & Engineering Chemistry Research., 30(1) (1991) 141–145. https://doi.org/10.1021/ie00049a022
Wang H, Zhang L, Han J, E W, DeePMD-kit: A deep learning package for many-body potential energy representation and molecular dynamics, Computer Physics Communications, 228 (2018) 178–84. https://doi.org/10.1016/j.cpc.2018.03.016
Bahri CNACZ, Al-Areqi WM, MIRuf FM, Majid AA, Characteristic of molten fluoride salt system LiF-BeF2 (Flibe) and LiF-NaF-KF (Flinak) as coolant and fuel carrier in molten salt reactor (MSR), AIP Conference Proceedings, 1799 (2017) 040008. https://doi.org/10.1063/1.4972932
Dracopoulos V, Gilbert B, Brrensen B, Photiadis GM, et al., Vibrational modes and structure of rare earth halide–alkali halide binary melts YBr3–ABr (A=Li, K, Cs) and YF3–KF, Journal of the Chemical Society, Faraday Transactions, 93 (1997) 3081–3088. https://doi.org/10.1039/a701864i
Photiadis GM, Brresen B, Papatheodorou GN, Vibrational modes and structures of lanthanide halide–alkali halide binary melts LnBr3–KBr (Ln=La, Nd, Gd) and NdCl3–ACl (A=Li, Na, K, Cs), Journal of the Chemical Society, Faraday Transactions, 94 (1998) 2605–2613. https://doi.org/10.1039/a802813c
Dracopoulos V, Gilbert B, Papatheodorou GN, Vibrational modes and structure of lanthanide fluoride–potassium fluoride binary melts LnF3–KF (Ln=La, Ce, Nd, Sm, Dy, Yb), Journal of the Chemical Society, Faraday Transactions, 94 (1998) 2601–2604. https://doi.org/10.1039/a802812e
Fukushima K, Yamoto H, Iwadate Y, Raman spectroscopic study of molten SmCl3–ACl systems (A=Li, Na, K), Journal of Alloys and Compounds, 290(1–2) (1999) 114–118. https://doi.org/10.1016/s0925-8388(99)00216-9
Stefanidaki E, Photiadis GM, Kontoyannis CG, Vik AF, et al., Oxide solubility and Raman spectra of NdF3–LiF–KF–MgF2–Nd2O3 melts, Journal of the Chemical Society, Dalton Transactions, (2002) 2302–2307. https://doi.org/10.1039/b111563b
Rollet A-L, Godier S, Bessada C, High temperature NMR study of the local structure of molten LaF3–AF (A = Li, Na, K and Rb) mixtures, Physical Chemistry Chemical Physics, 10 (2008) 3222–3228. https://doi.org/10.1039/b719158h
Bessada C, Rakhmatullin A, Rollet A-L, Zanghi D, High temperature NMR approach of mixtures of rare earth and alkali fluorides: An insight into the local structure, Journal of Fluorine Chemistry, 130(1) (2009) 45–52. https://doi.org/10.1016/j.jfluchem.2008.07.010
Deringer VL, Caro MA, Csányi G, Machine Learning Interatomic Potentials as Emerging Tools for Materials Science, Advanced Materials, 31(46) (2019) 1902765. https://doi.org/10.1002/adma.201902765
Mortazavi B, Zhuang X, Rabczuk T, Shapeev AV, Atomistic modeling of the mechanical properties: the rise of machine learning interatomic potentials, Materials Horizons, 10 (2023) 1956–1968. https://doi.org/10.1039/d3mh00125c
Mueller T, Hernandez A, Wang C, Machine learning for interatomic potential models, The Journal of Chemical Physics, 152(5) (2020) 050902. https://doi.org/10.1063/1.5126336
Hutter J, Iannuzzi M, Schiffmann F, VandeVondele J, Cp2k: atomistic simulations of condensed matter systems, Wiley Interdiscip, Rev.: Comput. Mol. Sci., 4(1) (2014) 15–25, https://doi.org/10.1002/wcms.1159
VandeVondele J, Hutter J, Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases, J Chem. Phys., 127(11) (2007) 114105. https://doi.org/10.1063/1.2770708
Perdew JP, Burke K, Ernzerhof M, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 77 (1996) 3865–3868. https://doi.org/10.1103/physrevlett.77.3865
Frandsen BA, Nickerson SD, Clark AD, Solano A, et al., The structure of molten FLiNaK, Journal of Nuclear Materials, 537 (2020) 152219. https://doi.org/10.1016/j.jnucmat.2020.152219
Nosé S, A unified formulation of the constant temperature molecular dynamics methods, J Chem. Phys., 81 (1984) 511–519. https://doi.org/10.1063/1.447334
Thompson AP, Aktulga HM, Berger R, Bolintineanu DS, et al., LAMMPS - a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales, Computer Physics Communications, 271 (2022) 108171. https://doi.org/10.1016/j.cpc.2021.108171
An X-H, Cheng J-H, Su T, Zhang P, Determination of thermal physical properties of alkali fluoride/carbonate eutectic molten salt, AIP Conference Proceedings, 1850(1) (2017) 070001. https://doi.org/10.1063/1.4984415
Janz GJ. Thermodynamic and Transport Properties for Molten Salts: Correlation Equations for Critically Evaluated Density Surface Tension Electrical Conductance and Viscosity Data. Part 17. New York: American Chemical Society and the American Institute of Physics; 1988. 309 p.
Salanne M, Simon C, Turq P, Madden PA, Heat-transport properties of molten fluorides: Determination from first-principles, J. Fluor. Chem., 130(1) (2009) 38–44, https://doi.org/10.1016/j.jfluchem.2008.07.013
Ingersoll DT, Forsberg CW, MacDonald PE. Trade Studies for the Liquid-Salt-Cooled Very High-Temperature Reactor: Fiscal Year 2006 Progress Report. Oak Ridge National Laboratory, Oak Ridge, Tennessee; 2007. 265 p.
Umesaki N, Iwamoto N, Tsunawaki Y, Ohno H, Furukawa K, Self-diffusion of lithium, sodium, potassium and fluorine in a molten LiF + NaF + KF eutectic mixture, Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 77 (1981) 169–175. https://doi.org/10.1039/f19817700169
Chesser R, Guo S, Zhang J, Electrochemical behavior of dysprosium and lanthanum in molten LiF-NaF-KF (Flinak) salt, Annals of Nuclear Energy, 120 (2018) 246–252. https://doi.org/10.1016/j.anucene.2018.05.045
Robertson SG, Wiser R, Yang W, Kang D, et al., The curious temperature dependence of fluoride molten salt thermal conductivity, Journal of Applied Physics, 131(22) (2022) 225102. https://doi.org/10.1063/5.0088059
Smirnov M, Khokhlov V, Filatov E, Thermal conductivity of molten alkali halides and their mixtures, Electrochimica Acta, 32(7) (1987) 1019–1026. https://doi.org/10.1016/0013-4686(87)90027-2
Gallagher RC, Birri A, Russell NG, Phan A-T, et al., Investigation of the thermal conductivity of molten LiF-NaF-KF with experiments, theory, and equilibrium molecular dynamics, Journal of Molecular Liquids, 361 (2022) 119151. https://doi.org/10.1016/j.molliq.2022.119151
Rudenko A, Redkin A, Il’ina E, Pershina S, et al., Thermal Conductivity of FLiNaK in a Molten State, Materials, 15(16) (2022) 5603. https://doi.org/10.3390/ma15165603
DOI: https://doi.org/10.15826/elmattech.2024.3.032
Copyright (c) 2024 Dmitry O. Zakiryanov

This work is licensed under a Creative Commons Attribution 4.0 International License.

