H/D isotope effects in the electrical conductivity of LaScO3-based solid state proton-conducting electrolyte
Abstract
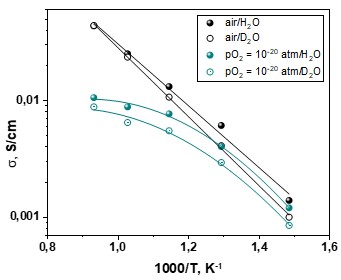
Electrical conductivity of a solid proton-conducting electrolyte with the composition of La0.9Sr0.1ScO3 is studied depending on temperature and oxygen partial pressure in the atmospheres, humidified with H2O and D2O. Isotope H/D effect in the electrical conductivity is observed at the temperatures below 700 °C in air conditions. For reductive atmosphere, the isotope effect is pronounced for the whole investigated temperature range. Based on the pO2-dependencies of electrical conductivity, the transport numbers of ions and electron holes are determined. The ionic transport numbers in the H2O-containing atmosphere are found to be higher than the corresponding values for the D2O-containing atmosphere for all investigated conditions. The possible role of protons (deuterons) in the oxygen ion transport is pointed out. The kinetic isotope effect is found to be predominant in the conditions under study.
Keywords
Full Text:
PDFReferences
Jiang X, Yin WJ, High-throughput computational screening of oxide double perovskites for optoelectronic and photocatalysis applications, J. Energy Chem., 57 (2021) 351–358. https://doi.org/10.1016/j.jechem.2020.08.046
Savchyn VP, Popov AI, Aksimentyeva OI, Klym H, et al., Cathodoluminescence characterization of polystyrene-BaZrO3 hybrid composites, Low Temp. Phys., 42 (2016) 597–600. https://doi.org/10.1063/1.4959020
Coondoo I, Alikin D, Abramov A, Figueiras FG, et al., Exploring the effect of low concentration of stannum in lead-free BCT-BZT piezoelectric compositions for energy related applications, J. Alloys Compd., 960 (2023) 170562. https://doi.org/10.1016/j.jallcom.2023.170562
Irvine J, Rupp JLM, Liu G, Xu X, et al., Roadmap on inorganic perovskites for energy applications, J. Phys. Energy, 3 (2021) 031502. https://doi.org/10.1088/2515-7655/abff18
Golkhatmi SZ, Asghar MI, Lund PD, A review on solid oxide fuel cell durability: latest progress, mechanisms, and study tools, Renew. Sustain. Energy Rev., 161 (2022) 112339. https://doi.org/10.1016/j.rser.2022.112339
Duan C, Huang J, Sullivan N, O’Hayre R, Proton–conducting oxides for energy conversion and storage, Appl. Phys. Rev., 7 (2020) 011314. https://doi.org/10.1063/1.5135319
Kim J, Sengodan S, Kim S, Kwon O, et al., Proton conducting oxides: a review of materials and applications for renewable energy conversion and storage, Renew. Sust. Energ. Rev., 109 (2019) 606–618. https://doi.org/10.1016/j.rser.2019.04.042
Zhang W, Hu YH, Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices, Energy Sci. Eng., 9 (2021) 984–1011. https://doi.org/10.1002/ese3.886
Kreuer KD, Proton-Conducting Oxides, Annu. Rev. Mater. Res., 33 (2003) 333–359 https://doi.org/10.1146/annurev.matsci.33.022802.091825
Rashid NLRM, Samat AA, Jais AA, Somalu MR, et al., Review on zirconate-cerate-based electrolytes for proton-conducting solid oxide fuel cell, Ceram. Int., 46 (2019) 6605–6615. https://doi.org/10.1016/j.ceramint.2019.01.045
Danilov NA, Starostina IA, Starostin GN, Kasyanova AV, et al., Fundamental understanding and applications of protonic Y and Yb-Coped Ba(Ce,Zr)O3 perovskites: state-of-the-art and perspectives, Adv. Energy Mater., 13 (2023) 2302175. https://doi.org/10.1002/aenm.202302175
Medvedev DA, Lyagaeva JG, Gorbova EV, Demin AK, et al., Advanced materials for SOFC application: Strategies for the development of highly conductive and stable solid oxide proton electrolytes, Prog. Mat. Sci., 75 (2016) 38–79. https://doi.org/10.1016/j.pmatsci.2015.08.001
Zając W, Rusinek D, Zheng K, Molenda J, Applicability of Gd-doped BaZrO3, SrZrO3, BaCeO3 and SrCeO3 proton conducting perovskites as electrolytes for solid oxide fuel cells, Cent. Eur. J. Chem., 11 (2013) 471–484. https://doi.org/10.2478/s11532-012-0144-9
Hossain MK, Chanda R, El-Denglawey A, Emrose T, et al., Recent progress in barium zirconate proton conductors for electrochemical hydrogen device applications: A review, Ceramics Int., 47 (2021) 23725–23748. https://doi.org/10.1016/j.ceramint.2021.05.167
Medvedev D, Murashkina A, Pikalova E, Demin A, et al., BaCeO3: materials development, properties and application, Prog. Mater. Sci., 60 (2014) 72–129. https://doi.org/10.1016/j.pmatsci.2013.08.001
Gorelov VP, Stroeva AY, Solid proton conducting electrolytes based on LaScO3, Russ. J. Electrochem., 48 (2012) 949–960. https://doi.org/10.1134/S1023193512100084
Nomura K, Takeuchi T, Tanase S, Kageyama H, et al., Proton conduction in (La0.9Sr0.1)MIIIO3–δ (MIII=Sc, In, and Lu) perovskites, Solid State Ionics, 154–155 (2002) 647–652. https://doi.org/10.1016/S0167-2738(02)00512-X
Kuzmin AV, Stroeva AYu, Gorelov VP, Novikova YuV, et al., Synthesis and characterization of dense proton-conducting La1–xSrxScO3–α ceramics, Int. J. Hydrog. Energy, 44 (2019) 1130–1138. https://doi.org/10.1016/j.ijhydene.2018.11.041
Lybye D, Poulsen FW, Mogensen M, Conductivity of A- and B-site doped LaAlO3, LaGaO3, LaScO3 and LaInO3 perovskites, Solid State Ionics, 128 (2000) 91–103. https://doi.org/10.1016/S0167-2738(99)00337-9
Stroeva AYu, Gorelov VP, Kuz'min AV, Antonova EP, et al., Phase composition and conductivity of La1−xSrxScO3−α (x = 0.01−0.20) under oxidative conditions, Russ. J. Electrochem., 48 (2012) 509–517. https://doi.org/10.1134/S1023193512050114
Belyakov SA, Lesnichyova AS, Plekhanov MS, Prinz N, et al., Dopant-induced changes of local structures for adjusting the hydration ability of proton conducting lanthanum scandates, J. Mater. Chem. A, 11 (2023) 19605–19618. https://doi.org/10.1039/D3TA03673A
Balakireva VB, Kuz’min AV, Gorelov VP, Ionic conductivity in the BaZr1−xYxO3−δ system (x = 0.02–0.2) in H2/H2O and D2/D2O atmospheres, Russ. J. Electrochem., 46 (2010) 749–753. https://doi.org/10.1134/S1023193510070050
Hossain MK, Yamamoto T, Hashizume K, Isotopic effect of proton conductivity in barium zirconates for various hydrogen-containing atmospheres, J. Alloys Compd., 903 (2022) 163957. https://doi.org/10.1016/j.jallcom.2022.163957
Li L, Nino JC, Proton-conducting barium stannates: Doping strategies and transport properties, Int. J. Hydrogen Energy, 38 (2013) 1598–1606. http://dx.doi.org/10.1016/j.ijhydene.2012.11.065
Ananyev MV, Farlenkov AS, Kurumchin EKh, Isotopic exchange between hydrogen from the gas phase and proton-conducting oxides: Theory and experiment, Int. J. Hydrog. Energy, 43 (2018) 13373–13382. https://doi.org/10.1016/j.ijhydene.2018.05.150
Tsidilkovski VI, Thermodynamic isotope effect H/D/T in proton-conducting oxides, Solid State Ionics, 162–163 (2003) 47–53. https://doi.org/10.1016/S0167-2738(03)00234-0
Antonova EP, Yaroslavtsev IYu, Bronin DI, Balakireva VB, et al., Peculiarities of electrical transfer and isotopic effects H/D in the proton-conducting oxide BaZr0.9Y0.1O3−δ, Russ. J. Electrochem., 46 (2010) 741–748. https://doi.org/10.1134/S1023193510070049
Nowick AS, Vaysleyb AV, Isotope effect and proton hopping in high-temperature protonic conductors, Solid State Ionics, 97 (1997) 17–26. https://doi.org/10.1016/S0167-2738(97)00081-7
Bonanos N, Huijser A, Poulsen FW, H/D isotope effects in high temperature proton conductors, Solid State Ionics, 275 (2015) 9–13. http://dx.doi.org/10.1016/j.ssi.2015.03.028
Farlenkov AS, Putilov LP, Ananyev MV, Antonova EP, et al., Water uptake, ionic and hole transport in La0.9Sr0.1ScO3–δ, Solid State Ionics, 306 (2017) 126–136. https://doi.org/10.1016/j.ssi.2017.04.013
Basbus JF, Arce MD, Napolitano FR, Troiani HE, et al., Revisiting the crystal structure of BaCe0.4Zr0.4Y0.2O3−δ proton conducting perovskite and its correlation with transport properties, ACS Appl. Energy Mater., 3 (2020) 2881–2892. https://dx.doi.org/10.1021/acsaem.9b02498
Stroeva AYu, Gorelov VP, Nature of conductivity of perovskites La1–xSrxScO3–α (x = 0.01–0.15) under oxidative and reducing conditions, Russ. J. Electrochem., 48 (2012) 1079–1085. https://doi.org/10.1134/S1023193512110158
Okuyama Y, Kozai T, Ikeda S, Matsuka M, et al., Incorporation and conduction of proton in Sr-doped LaMO3 (M = Al, Sc, In, Yb, Y), Electrichim. Acta, 125 (2014) 443–449. https://doi.org/10.1016/j.electacta.2014.01.113
Antonova EP, Gordeev EV, Fedorova KA, Features of electrical transport and H/D isotope effects in the proton-conducting electrolyte BaCe0.7Zr0.1Y0.1Yb0.1O3–δ, Solid State Sciences, 154 (2024) 107625. https://doi.org/10.1016/j.solidstatesciences.2024.107625
DOI: https://doi.org/10.15826/elmattech.2024.3.042
Copyright (c) 2024 Ekaterina P. Antonova

This work is licensed under a Creative Commons Attribution 4.0 International License.

