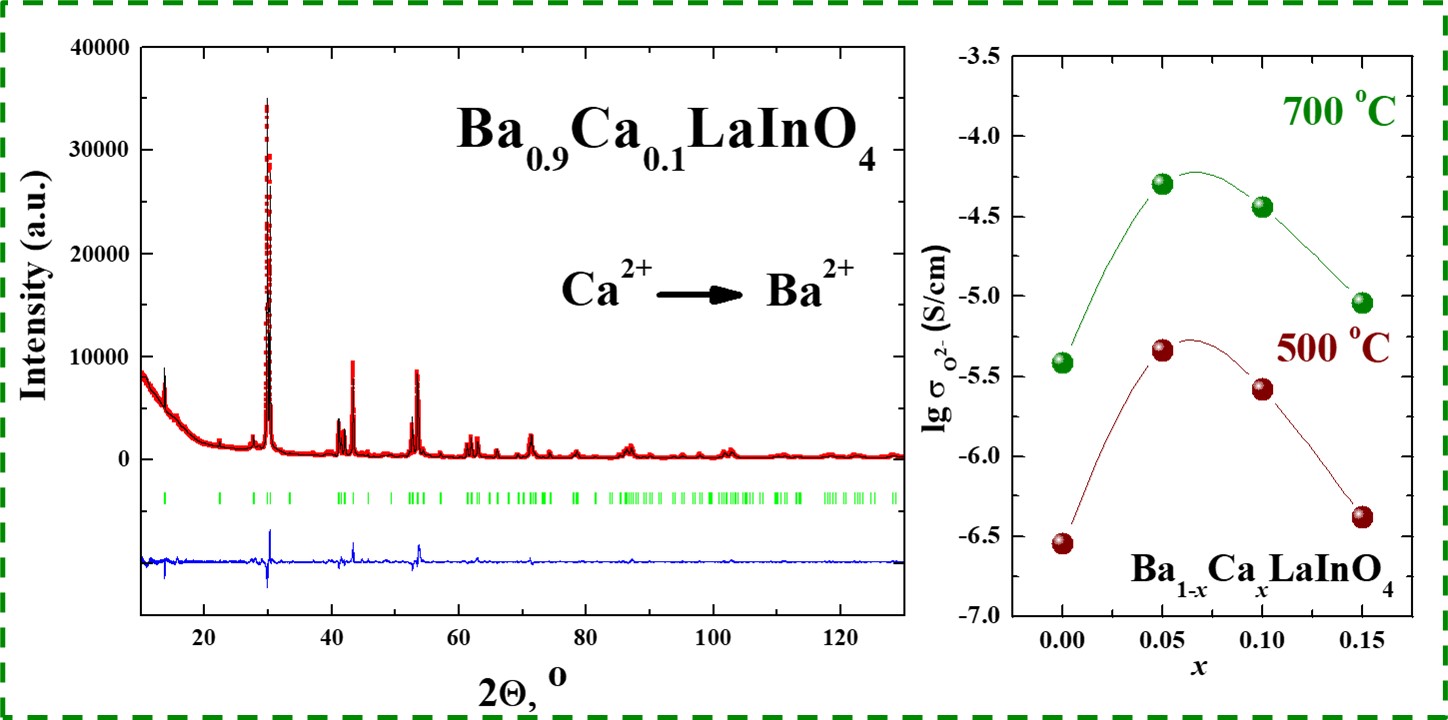
Oxygen-ionic transport in the novel Ca-doped complex oxides based on BaLaInO4
Abstract
Keywords
Full Text:
PDFReferences
Shu DY, Deutz S, Winter BA, Baumgärtner N, The role of carbon capture and storage to achieve net-zero energy systems: trade-offs between economics and the environment, Ren. Sust. Ener. Rev., 178(6396) (2023) 113246. https://doi.org/10.1016/j.rser.2023.113246
Davis SJ, Caldeira K, Matthews HD, Future CO2 emissions and climate change from existing energy infrastructure, Science, 329(5997) (2010) 1330–1333. https://doi.org/10.1126/science.1188566
Tyquin E, Weeks CS, Mehta A, Newton C, Delivering an energy export transition: Impact of conflicting and competing informational contexts on public acceptance of Australia’s hydrogen export industry, Int. J. Hydrogen Energy, 61 (2024) 226–237. https://doi.org/10.1016/j.ijhydene.2024.02.185
Krause J, Yugo M, Samaras Z, Edwards S, et al., Well-to-wheels scenarios for 2050 carbon-neutral road transport in the EU, J. Clean. Prod., 443 (2024) 141084. https://doi.org/10.1016/j.jclepro.2024.141084
Younas M, Shafique S, Hafeez A, Javed F, et al., An overview of hydrogen production: current status, potential, and challenges, Fuel, 31615 (2022) 123317. https://doi.org/10.1016/j.fuel.2022.123317
Singh M, Zappa D, Comini E, Solid oxide fuel cell: Decade of progress, future perspectives and challenges, Int. J. Hydrogen Energy, 46(54) (2021) 27643–27674. https://doi.org/10.1016/j.ijhydene.2021.06.020
Xu Q, Guo Z, Xia L, He Q, et al., A comprehensive review of solid oxide fuel cells operating on various promising alternative fuels, Energy Conversion and Management, 253 (2022) 115175. https://doi.org/10.1016/j.enconman.2021.115175
Peng J, Huang J, Wu X, Xu Y, et al., Solid oxide fuel cell (SOFC) performance evaluation, fault diagnosis and health control: A review, Journal of Power Sources, 505 (2021) 230058. https://doi.org/10.1016/j.jpowsour.2021.230058
Parente C, Teixeira F, Cerdeira J, ’Stakeholders’ perceptions of hydrogen and reflections on energy transition governance, Energy. Sustain. Soc., 14 (2024) 1–19. https://doi.org/10.1186/s13705-023-00429-w
Vansh M, Siddharth S, Mudit KB, Mohit V, Comparative study and analysis between Solid Oxide Fuel Cells (SOFC) and Proton Exchange Membrane (PEM) fuel cell – A review, Materials today: Proceedings, 47(10) (2021) 2270–2275. https://doi.org/10.1016/j.matpr.2021.04.203
Norby T, Widerøe M, Glöckner R, Larring Y, Hydrogen in oxides, Dalton Transactions, 19 (2004) 3012–3018. https://doi.org/10.1039/B403011G
Syafkeena N, Affandi M, Osman N, Short review on global trends in SOFC scenario and future perspective, Materials today: Proceedings, 66(10) (2022) 3981–3984. https://doi.org/10.1016/j.matpr.2022.04.824
Kong W, Han Zh, Lu S, Gao X, A novel interconnector design of SOFC, Int. J. Hydrogen Energy, 45(39) (2020) 20329–20338. https://doi.org/10.1016/j.ijhydene.2019.10.252
Sahli Y, Moussa HB, Zitouni B, Optimization study of the produced electric power by SOFCs, Int. J. Hydrogen Energy, 44(39) (2019) 22445–22454. https://doi.org/10.1016/j.ijhydene.2018.08.162
Matsuzaki K, Saito K, Ikeda Y, Nambu Y, et al., High Proton Conduction in the Octahedral Layers of Fully Hydrated Hexagonal Perovskite-Related Oxides, J. Am. Chem. Soc., 146(27) (2024) 18544–18555. https://doi.org/10.1021/jacs.4c04325
Fop S, Dawson JA, Fortes AD, Ritter C, McLaughlin AC, Hydration and ionic conduction mechanisms of hexagonal perovskite derivative, Chemistry of Materials, 33(12) (2021) 4651–4660. https://doi.org/10.1021/acs.chemmater.1c01141
Tarasova N, Animitsa I, Galisheva A, Medvedev D, Layered and hexagonal perovskites as novel classes of proton-conducting solid electrolytes: A focus review, Electrochem. Mater. Technol., 1 (2022) 20221004. https://doi.org/10.15826/elmattech.2022.1.004
Tarasova N, Galisheva A, Animitsa I, Korona D, et. al., Protonic transport in layered perovskites BaLanInnO3n+1 (n = 1, 2) with Ruddlesden-Popper structure, Appl. Sci., 12(8) (2022) 4082. https://doi.org/10.3390/app12084082
Tarasova N, Animitsa I, Galisheva A, Pryakhina V, Protonic transport in the new phases BaLaIn0.9M0.1O4.05 (M = Ti, Zr) with Ruddlesden-Popper structure, Solid State Sciences, 101 (2020) 106121. https://doi.org/10.1016/j.solidstatesciences.2020.106121
Tarasova N, Galisheva A, Animitsa I, Anokhina I, Novel mid-temperature Y3+ → In3+ doped proton conductors based on the layered perovskite BaLaInO4, Ceramics International, 48(11) (2022) 15677–15685. https://doi.org/10.1016/j.ceramint.2022.02.102
Shannon RD, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Cryst. A, 32 (1976) 751–767. https://doi.org/10.1107/S0567739476001551
Tarasova N, Galisheva A, Animitsa I, Korona D, Incorporation and conduction of protons in Ca, Sr, Ba-doped BaLaInO4 with Ruddlesden-Popper structure, Materials, 12(10) (2019) 1668–1682. https://doi.org/10.3390/ma12101668
DOI: https://doi.org/10.15826/elmattech.2025.4.051
Copyright (c) 2025 Natalia A. Tarasova, Ekaterina V. Abakumova, Tamara A. Kuznetsova

This work is licensed under a Creative Commons Attribution 4.0 International License.

