Influence of caprolactam on the tin electrodeposition on a dispersed carbon support and preparation of Pt/(SnO2/C) catalysts
Abstract
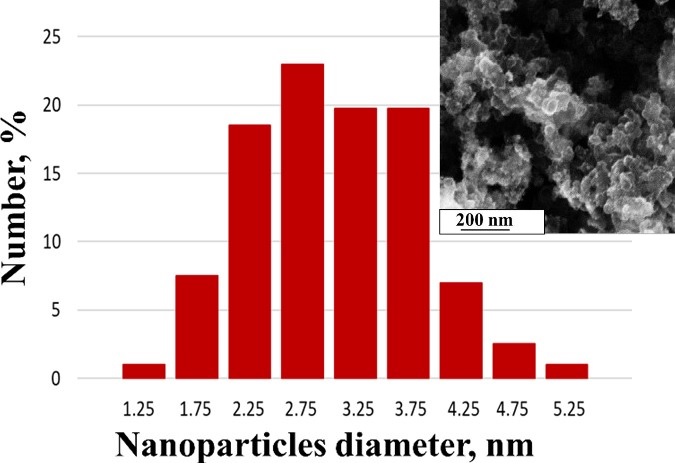
Composite SnO2/C materials obtained by electrodeposition of tin on Vulcan XC72 particles were used to fabricate a platinum catalyst for the oxygen electroreduction reaction (ORR). Thermogravimetry and X-ray diffraction methods made it possible to determine the composition of materials and the size of platinum and tin oxide crystallites. The use of the SnO2/C composite obtained in the presence of e-caprolactam (CPL) as a support made it possible to increase the electrochemically active surface area (ESA) of platinum and the mass activity of the Pt/SnO2/C catalyst in ORR.
Keywords
Full Text:
PDFReferences
Thompsett D, Catalysts for the proton exchange membrane fuel cell, Eds: Vielstich, W., N.Y.: Wiley & Sons, 3 (2003) 6–23. https://doi.org/10.1201/9781420041552
Zhang J, Wang X, Wu C, Preparation and characterization of Pt/C catalysts for PEMFC cathode: effect of different reduction methods, React. Kinet. Catal. Lett. 83 (2004) 229–236. https://doi.org/10.1023/B:REAC.0000046081.96554.ae
Chen J, Jiang C, Yang X, Feng L, et al., Studies on how to obtain the best catalytic activity of Pt/C catalyst by three reduction routes for methanol electro-oxidation, Electrochem. Comm. 13 (2011) 314–316. https://doi.org/10.1016/j.elecom.2011.01.012
Prabhuram J, Zhao T, Wong C, Guo J, Synthesis and physical/electrochemical characterization of Pt/C nano-catalyst for polymer electrolyte fuel cells, J. Pow. Sources 134 (2004) 1–6. https://doi.org/10.1016/j.jpowsour.2004.02.021
Guterman VE, Novomlinskij IN, Skibina LM, Mauer DK, Method for Obtaining Nanostructural Material of tin Oxide on Basis of Carbon. Russian Federation patent RUS 2656914. 2018 June 7.
Novomlinskij IN, Volochaev VА, Tsvetkova GG, Guterman VЕ, New electrochemical method for the preparation of Pt/C nanostructured materials, Cond. Matter Interphas. 19 (2017) 112-119. https://doi.org/10.1007/s00706-019-2383-3
Kuriganova AB, Leontyeva DV, Ivanov S, et al., Electrochemical dispersion technique for preparation of hybrid MOx–C supports and Pt/MOx–C electrocatalysts for low-temperature fuel cells, J. Appl. Electrochem. 46 (2016) 1245–1260. https://doi.org/10.1007/s10800-016-1006-5
Zhang K, Feng C, He B, Dong H, Dai W, et al., An advanced electrocatalyst of Pt decorated SnO2/C nanofibers for oxygen reduction reaction, J. Electroanalyt. Chem. 781 (2016) 198–203. https://doi.org/10.1016/j.jelechem.2016.11.002
Dahl PI, Barnett AO, Monterrubio FA, Colmenares LC, The use of tin oxide in fuel cells, In: Tin oxide materials, Elsevier. 781 (2020) 379–410. https://doi.org/10.1016/B978-0-12-815924-8.00013-X
Santos AO, Silva JCM, Antoniassi RM, Ponzio EA, et al., The format electrooxidation on Pt/C and PtSnO2/C nanoparticles in alkaline media: The effect of morphology and SnO2 on the platinum catalytic activity, Int. J. Hydr. Ener. 45 (2020) 33895–33905. https://doi.org/10.1016/j.ijhydene.2020.08.165
Mensharapov RM, Ivanova N A, Spasov D D, Kukueva EV, et al., Carbon-supported Pt-SnO2 catalysts for oxygen reduction reaction over a wide temperature range: Rotating disk electrode study, Catalysts. 11 (2021) 1469. https://doi.org/10.3390/catal11121469
Hussain S, Kongi N, Erikson H, Rähn M, et al., Platinum nanoparticles photo-deposited on SnO2-C composites: An active and durable electrocatalyst for the oxygen reduction reaction, Electrochim. Acta. 316 (2019) 162−172. https://doi.org/10.1016/j.electacta.2019.05.104
Chao G, An X, Zhang L, Tian J, et al., Electron-rich platinum electrocatalysts supported onto tin oxides for efficient oxygen reduction, Composites Commun. 24 (2021) 100603. https://doi.org/10.1016/j.coco.2020.100603
Shen X, Nagai T, Yang F, Zhou L, et al., Dual-site cascade oxygen reduction mechanism on SnOx/Pt–Cu–Ni for promoting reaction kinetics, J. Am. Chem. Soc. 141 (2019) 9463–9467. https://doi.org/10.1021/jacs.9b02286
Madhu SS, Ruying L, Mei C, Xueliang S, High electrocatalytic activity of platinum nanoparticles on SnO2 nanowire-based electrodes, Electrochem. Solid-State Lett. 10 (2007) B130-B133. https://doi.org/10.1149/1.2745632
Lee JH, Park SJJ, Preparation of spherical TiO2/SnO2 powders by ultrasonic spray pyrolysis and its spinodal decomposition, Am. Ceram. Soc. 76 (1993) 254–258. https://doi.org/10.1007/BF00179220
Williams G, Coles GSV, Gas sensing properties of nanocrystalline metal oxide powders produced by a laser evaporation technique, J. Mater. Chem. 8 (1998) 1657–1664. https://cronfa.swan.ac.uk/Record/cronfa26914
Willett MJ, Burganos VN, Tsakiroglou CD, Payatakes AC, Nanocrystalline oxides for gas, sensing, Sens. Actuators. (1998) B 53–76. https://doi.org/10.1007/0-306-47609-6_7
Zhang J, Gao L, Synthesis of SnO2 Nanoparticles by the sol-gel method from granulated tin, Chem. Lett. 32 (2003) 458–459. https://doi.org/10.1246/cl.2003.458
De Monredon S, Cellot A, Ribot F, Sanchez C, et al., Synthesis and characterization of crystalline tin oxidenanoparticles, J. Mater. Chem. 12 (2002) 2396–2400. https://doi.org/10.1039/B203049G
Song KC, Kang Y, Preparation of high surface area tin oxide powders by a homogeneous precipitation method, Mater. Lett. 42 (2000) 283–289. https://doi.org/10.1016/S0167-577X(99)00199-8
Meiling D, Ming H, Dong Li, Wangting L, et al., SnO2 nanocluster supported Pt catalyst with high stability for proton exchangemembrane fuel cells, Electrochim. Acta. 92 (2013) 468–473. https://doi.org/10.1016/j.electacta.2013.01.070
Skibina LM, Mauer DK, Sokolenko, The effect of cyclic lactams and their structural analogs on the surface morphology, coating properties, and electroreduction kinetics of Cu(II) ions, Protec. Metals Phys. Chem. Surf. 54 (2018) 624–631. https://doi.org/10.1134/S2070205118030164
Skibina LM, Kuznetsov VV, Sukholentsev EA, The effect of the ε-caprolactam concentration on the electrodeposition of nickel–polymer coatings, Protec. Metals. 37 (2001) 159–162. https://doi.org/10.1023/A:1010330222922
Novomlinski IN, Tabachkova NY, Safronenko OI, Guterman VE, Novel electrochemical method for the preparation of Pt/C nanostructured material, Monatshefte Für Chemie - Chemical Monthly. 150 (2019) 631–637. https://doi.org/10.1007/s00706-019-2383-3
Shinozaki K, Zack JW, Pylypenko S, Pivovar BS, et al., Oxygen reduction reaction measurements on platinum electrocatalysts utilizing rotating disk electrode technique, J. Electrochem. Soc. 162 (2015) 1384–1396. https://doi.org/10.1149/2.1071509jes
Suryanarayana C, Norton MG, X-ray diffraction: a practical approach, Springer Science & Business Media. (2013) 273. https://doi.org/10.1007/978-1-4899-0148-4
Gražulis S, Daškevič A, Merkys A, Chateigner, et al., Crystallography Open Database (COD): an open-access collection of crystal structures and platform for world-wide collaboration Nucleic Acids Research, 40 (2012) D420–D427. https://doi.org/10.1093/nar/gkr900
Klug HP, Alexander LE, X-Ray Diffraction procedures: for polycrystalline and amorphous materials, 2nd Edition John Wiley: New York USA, (1974) 992.
Novomlinskiy IN, Danilenko MV, Safronenko OI, Tabachkova NY, et al., Influence of the Sn-oxide-carbon carrier composition on the functional characteristics of deposited platinum electrocatalysts, Electrocatalys. 12 (2021) 489–498. https://doi.org/10.1007/s12678-021-00649-8
Savinova DV, Molodkina EB, Danilov AI, Polukarov YM, Surface and subsurface oxygen on platinum in a perchloric acid solution, Rus. J. Electrochem. 40 (2004) 683–687. https://doi.org/10.1023/B:RUEL.0000035248.39620.f
DOI: https://doi.org/10.15826/elmattech.2023.2.009
Copyright (c) 2023 Dmitry K. Mauer, Sergey V. Belenov

This work is licensed under a Creative Commons Attribution 4.0 International License.

