Mechanism of interaction between zirconium dioxide and fluoride melts
Abstract
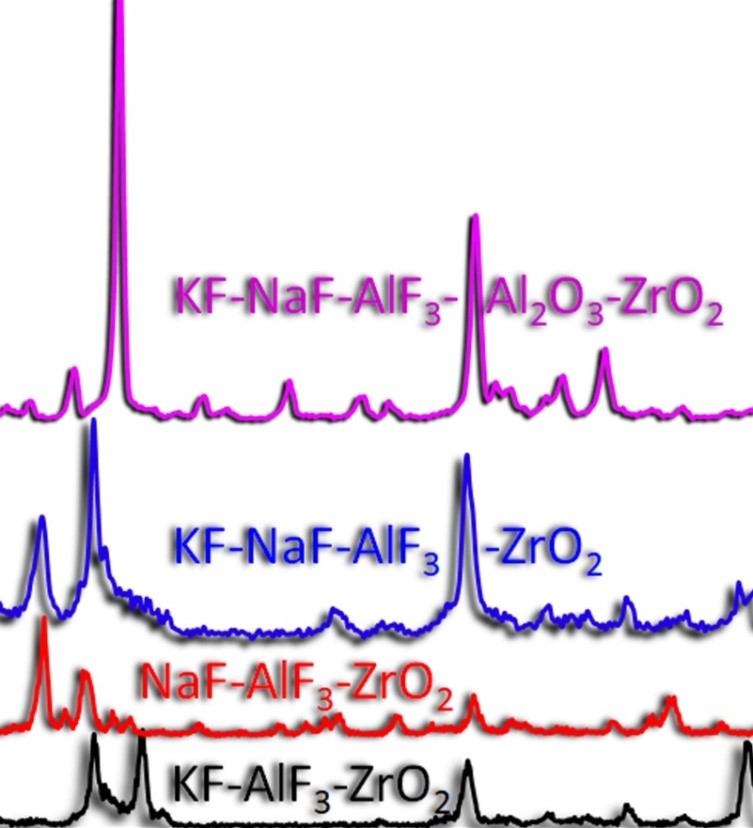
In this work, the mechanism of interaction between zirconium dioxide and fluoride melts was studied. Dissolution rates and maximum permissible oxide contents in the KF-AlF3 and NaF-AlF3 melts were determined under natural convection conditions at a temperature of 750 °C. The results of solubility measurement show that the limiting content of ZrO2 in the KF-AlF3 melt ([KF]/[AlF3] = 1.3) is 0.69 ± 0.10 mol. %, and in the NaF-AlF3 melt ([NaF]/[AlF3] = 1.3) at 800 °C it is 0.54 ± 0.04 mol. %. The calculated dissolution rate of zirconium oxide under these conditions in melts based on potassium fluoride and sodium fluoride is 0.022 mol/min and 0.017 mol/min, respectively. The influence of the cationic composition of the medium and the concentrations of additives on the liquidus temperatures of the melts was studied. It has been established that the increase in liquidus and solidus temperatures is facilitated by the addition of zirconium and aluminium oxides, as well as by the replacement of potassium fluoride with sodium fluoride while maintaining the molar ratio of the replaced cations. Analysis of thermal effects by differential scanning calorimetry (DSC) shows that, regardless of the molar ratio of the components, the onset of the thermal effect in melts based on potassium fluoride occurs at a temperature of 542 °C and in the systems with sodium fluoride additives at a temperature of 597 °C. Presumably, this dependence is associated with the melting of the KAlF4 and K2NaAl3F12 phases formed in the melts based on KF-AlF3 and KF-NaF-AlF3, respectively. It has been established that the melting of the KF-AlF3 mixture is accompanied with a mass loss of up to 1.3 wt. %, which is associated with partial evaporation of KAlF4.
Keywords
References
Tkacheva O, Redkin A, Kataev A, Rudenko A, et al., Novel molten salts media for production of functional materials, MATEC Web of Conferences, 67 (2016) 1–6. https://doi.org/10.1051/matecconf/20166706044
Pershin PS, Kataev AA, Filatov AA, Suzdaltsev AV, et al., Synthesis of Al-Zr alloys via ZrO2 aluminum-thermal reduction in KF-AlF3-based melts, MMTB, 48 (2017) 1962–1969. https://doi.org/10.1007/s11663-017-0976-y
Pershin PS, Suzdaltsev AV, Zaikov YuP, Synthesis of silumins in KF–AlF3–SiO2 melt, JES, 163(5) (2016) 167–170. https://doi.org/10.1149/2.0521605jes
Suzdaltsev AV, Khramov AP, Zaikov YuP, Carbon electrode for electrochemical studies in cryolite-alumina melts at 700–960 °C, Russ. J Electrochem., 48 (2012) 1141–1152. https://doi.org/10.1134/S1023193512120117
Apisarov A, Juan B, Dedyukhin A, Galan L, et al., Reduction of the operating temperature of aluminum electrolysis: Low temperature electrolyte, Light Met. (2012) 783–786. https://doi.org/10.1002/9781118359259.ch135
Apisarov A, Dedyukhin A, Tinghaev P, Redkin A, et al., Liquidus Temperatures of Cryolite Melts With Low Cryolite Ratio, MMTB, 42 (2010) 236–242. https://doi.org/10.1007/s11663-010-9462-5
Danielik V, Hives J, Low-Melting Electrolyte for Aluminum Smelting, J. of Chem. and Eng. Data, 35(47) (2004) 1414–1417. https://doi.org/10.1002/chin.200447013
Peng J, Wei Z, Di Y, Wang Y, et al., Alumina Solubility in NaF-KF-LiF-AlF3-Based Low-Temperature Melts, JOM, 72 (2020) 239–246. https://doi.org/10.1007/s11837-019-03873-2
Kirik SD, Zaitseva YuN, Leshok DYu, Samoilo AS, et al., NaF-KF-AlF3 System: Phase Transition in K2NaAl3F12 Ternary Fluoride, Inorg. Chem., 54(12) (2015) 5960–5970. https://doi.org/10.1021/acs.inorgchem.5b00772
Picard GS, Bouyer FC, Leroy M, Bertaud Y, et al., Structures of oxyfluoraluminates in molten cryolite-alumina mixtures investigated by DFT-based calculations, J. Molec. Struct., 368 (1996) 67–80. https://doi.org/10.1016/S0166-1280(96)90539-4
Nekrasov VN, Suzdaltsev AV, Limanovskaya O, Khramov AP, et al., Theoretical and experimental study of anode process on carbon in KF-AlF3-Al2O3 melts, Electrochim. Acta., 75 (2012) 296–304. https://doi.org/10.1016/j.electacta.2012.05.007
Filatov AA, Pershin PS, Suzdaltsev AV, Nikolaev AYu, et al., Synthesis of Al-Zr Master Alloys via the Electrolysis of KF-NaF-AlF3-ZrO2 Melts, JES, 165(2) (2018) 28–34. https://doi.org/10.1149/2.0571802JES
Suzdaltsev AV, Filatov AA, Nikolaev AYu, Pankratov AA, et al., Extraction of Scandium and Zirconium from their oxides during the electrolysis of oxide–fluoride melts, Rus. Met. (Metally), 2018 (2018) 133–138. https://doi.org/10.1134/S0036029518020180
Filatov AA, Suzdaltsev AV, Nikolaev AYu, Zaikov YuP, Kinetics of electrical release of zirconium and aluminum from KF–AlF3–ZrO2 melts, Melts, 3 (2019) 287–304. (In Russian). https://doi.org/10.1134/S0235010619030046
Suzdaltsev AV, Pershin PS, Filatov AA, Nikolaev AYu, et al., Review-Synthesis of Aluminum Master Alloys in Oxide-Fluoride Melts: A Review, JES, 167(10) (2020) 102503. https://doi.org/10.1149/1945-7111/ab9879
Filatov AA., Suzdaltsev AV, Zaikov YuP, Comparative analysis of modern methods for producing Al – Zr alloys, Tsvetnye Metally: Metal processing, 4 (2021) 78–86. (In Russian). https://doi.org/10.17580/tsm.2021.04.13
Filatov AA, Suzdaltsev AV, Zaikov YuP, Modifying Ability of an Al–Zr Master Alloy, Rus. Met. (Metally), 2021 (2021) 1036–1039. https://doi.org/10.1134/S0036029521080073
Filatov AA, Suzdaltsev AV, Zaikov YuP, Production of Al-Zr Master Alloy by Electrolysis of the KF-NaF-AlF3-ZrO2 Melt: Modifying Ability of the Master Alloy, MMTB, 52(6) (2021) 4206–4214. https://doi.org/10.1007/s11663-021-02340-1
DOI: https://doi.org/10.15826/elmattech.2024.3.035
Copyright (c) 2024 Aleksandr A. Filatov

This work is licensed under a Creative Commons Attribution 4.0 International License.

