Structural stability and features of electrical and electrochemical behavior under reducing conditions of Pr0.4Sr0.6Co0.2Fe0.7Nb0.1O3–δ material for the symmetrical SOFCs
Abstract
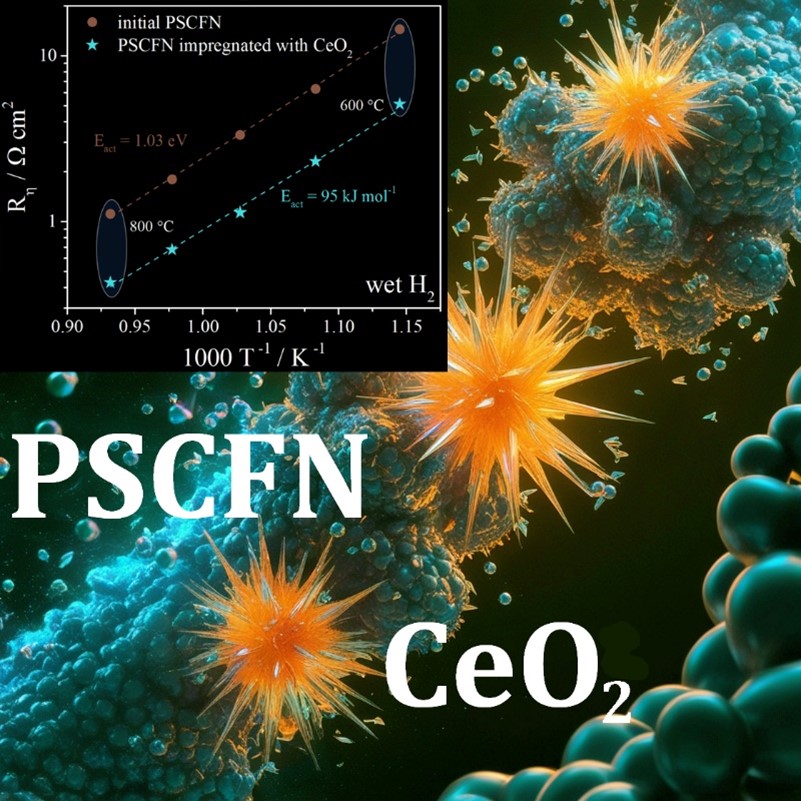
In this study, a performance of the complex oxide composition of Pr0.4Sr0.6Co0.2Fe0.7Nb0.1O3–δ was investigated from the viewpoint of its use as a fuel or symmetrical electrode for the electrochemical devices with a LaGaO3-based solid electrolyte. The results show that the above-mentioned oxide can be obtained as a single-phase composition using solid-phase synthesis with a final annealing temperature of 1150 °C. It has been shown that the oxide retains satisfactory stability at 800 °C in an atmosphere of 5 % H2 + Ar, only a minor amount, presumably of Co-Fe alloy, has been detected. The electrical conductivity of the oxide in wet hydrogen exhibits a linear semiconductor-type behavior with a conductivity value of 7 S · cm–1 at 800 °C. The polarization resistance of the Pr0.4Sr0.6Co0.2Fe0.7Nb0.1O3–δ electrode in wet hydrogen atmosphere reaches approximately 1.09 Ω · cm2 at 800 °C, which is a relatively high value for the electrodes of electrochemical devices. A significant reduction in resistance down to 0.43 Ω · cm2 is observed for the electrode activated with impregnated ceria. It has been demonstrated that the observed decrease in resistance is due to the expansion of the area of the electrochemical reaction without changing its mechanism. The long-term tests with a duration of about 220 h at 800 °C in a wet hydrogen atmosphere demonstrate satisfactory stability of the electrochemical activity of the Pr0.4Sr0.6Co0.2Fe0.7Nb0.1O3–δ electrode, which can be considered as a promising electrode for intermediate temperature electrochemical devices, including those of symmetrical design.
Keywords
Full Text:
PDFReferences
Singh M, Zappa D, Comini E, Solid oxide fuel cell: Decade of progress, future perspectives and challenges, Int. J. Hydrog. Energy, 46(54) (2021) 27643–27674. https://doi.org/10.1016/j.ijhydene.2021.06.020
Demin AK, Bronin DI, Solid state electrochemical devices for hydrogen energy, Electrochem. Mater. Technol., 2 (2023) 20232016. https://doi.org/10.15826/elmattech.2023.2.016
Jiang S, Advances and Challenges of Intermediate Temperature Solid Oxide Fuel Cells: A Concise Review, J. Electrochem., 18(6) (2012) 1. https://doi.org/10.61558/2993-074X.2617
Sadykov VA, Eremeev NF, Sadovskaya EM, et al., Design of materials for solid oxide fuel cells, permselective membranes, and catalysts for biofuel transformation into syngas and hydrogen based on fundamental studies of their real structure, transport properties, and surface reactivity, Curr. Opin. Green Sustain. Chem., 33 (2022) 100558. https://doi.org/10.1016/j.cogsc.2021.100558
Mah JCW, Muchtar A, Somalu MR, Ghazali MJ, Metallic interconnects for solid oxide fuel cell: A review on protective coating and deposition techniques, Int. J. Hydrog. Energy., 42(14) (2017) 9219–9229. https://doi.org/10.1016/j.ijhydene.2016.03.195
Sharifzadeh M, Chen W, Triulzi G, et al., Design and operation of solid oxide fuel cell systems: challenges and future research directions. In: Design and Operation of Solid Oxide Fuel Cells. Elsevier; 2020. pp 445–463.
Zhu Q, Peng L, Zhang T, Stable Glass Seals for Intermediate Temperature (IT) SOFC Applications. In: Kuang K, Easler K (eds) Fuel Cell Electronics Packaging. Springer US, Boston, MA; 2007. pp 33–60.
Mendonça C, Ferreira A, Santos DMF, Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies, Fuels, 2(4) (2021) 393–419. https://doi.org/10.3390/fuels2040023
Dwivedi S, Solid oxide fuel cell: Materials for anode, cathode and electrolyte, Int. J. Hydrog. Energy, 45(44) (2020) 23988–24013. https://doi.org/10.1016/j.ijhydene.2019.11.234
Istomin SY, Antipov EV, Cathode materials based on perovskite-like transition metal oxides for intermediate temperature solid oxide fuel cells, Russ. Chem. Rev., 82 (2013) 686–700. https://doi.org/10.1070/RC2013v082n07ABEH004390
Xu H, Han Y, Zhu J, et al., Status and progress of metal-supported solid oxide fuel cell: Towards large-scale manufactory and practical applications, Energy Rev., 3(1) (2024) 100051. https://doi.org/10.1016/j.enrev.2023.100051
Pikalova EYu, Kalinina EG, Place of electrophoretic deposition among thin-film methods adapted to the solid oxide fuel cell technology: A short review, Int. J. Energy Prod. Manag., 4(1) (2019) 1–27. https://doi.org/10.2495/EQ-V4-N1-1-27
Dunyushkina LA, Solid Oxide Fuel Cells with a Thin Film Electrolyte: A Review on Manufacturing Technologies and Electrochemical Characteristics, Electrochem. Mater. Technol., 1 (2022) 20221006. https://doi.org/10.15826/elmattech.2022.1.006
Pikalova EYu, Kalinina EG, Solid oxide fuel cells based on ceramic membranes with mixed conductivity: improving efficiency, Russ. Chem. Rev., 90 (2021) 703–749. https://doi.org/10.1070/RCR4966
Antonova EP, Proton-conducting oxides based on LaScO3: structure, properties and electrochemical applications. A focus review, Electrochem. Mater. Technol., 2 (2023) 20232021. https://doi.org/10.15826/elmattech.2023.2.021
Filonova E, Medvedev D, Recent Progress in the Design, Characterisation and Application of LaAlO3- and LaGaO3-Based Solid Oxide Fuel Cell Electrolytes, Nanomaterials, 12 (2022) 1991. https://doi.org/10.3390/nano12121991
Tahir NNM, Baharuddin NA, Samat AA, Osman N, Somalu MR, A review on cathode materials for conventional and proton-conducting solid oxide fuel cells, J. Alloys Compd., 894 (2022) 162458. https://doi.org/10.1016/j.jallcom.2021.162458
Kaur P, Singh K, Review of perovskite-structure related cathode materials for solid oxide fuel cells, Ceram. Int., 46(5) (2020) 5521–5535. https://doi.org/10.1016/j.ceramint.2019.11.066
Baratov S, Filonova E, Ivanova A, et al., Current and further trajectories in designing functional materials for solid oxide electrochemical cells: A review of other reviews, J. Energy Chem., 94 (2024) 302–331. https://doi.org/10.1016/j.jechem.2024.02.047
Yáng Z, Martynczuk J, Efimov K, et al., Oxygen-Vacancy-Related Structural Phase Transition of Ba0.8Sr0.2Co0.8Fe0.2O3-δ, Chem. Mater., 23 (2011) 3169–3175. https://doi.org/10.1021/cm200373r
Pan Z, Liu Q, Zhang L, et al., Effect of Sr Surface Segregation of La0.6Sr0.4Co0.2Fe0.8O3-δ Electrode on Its Electrochemical Performance in SOC, J. Electrochem. Soc., 162 (2015) F1316–F1323. https://doi.org/10.1149/2.0371512jes
Koo B, Kim K, Kim JK, et al., Sr Segregation in Perovskite Oxides: Why It Happens and How It Exists, Joule, 2(8) (2018) 1476–1499. https://doi.org/10.1016/j.joule.2018.07.016
Safian SD, Abd Malek NI, Jamil Z, et al., Study on the surface segregation of mixed ionic‐electronic conductor lanthanum‐based perovskite oxide La1-xSrxCo1-yFeyO3-δ materials, Int. J. Energy Res., 46 (2022) 7101–7117. https://doi.org/10.1002/er.7733
Shah N, Xu X, Love J, et al., Mitigating thermal expansion effects in solid oxide fuel cell cathodes: A critical review, J. Power Sources, 599 (2024) 234211. https://doi.org/10.1016/j.jpowsour.2024.234211
Lyagaeva J, Medvedev D, Pikalova E, et al., A detailed analysis of thermal and chemical compatibility of cathode materials suitable for BaCe0.8Y0.2O3−δ and BaZr0.8Y0.2O3−δ proton electrolytes for solid oxide fuel cell application, Int. J. Hydrog. Energy, 42(3) (2024) 1715–1723. https://doi.org/10.1016/j.ijhydene.2016.07.248
Pikalova EYu, Guseva EM, Filonova EA, Short review on recent studies and prospects of application of rare-earth-doped La2NiO4+δ as air electrodes for solid-oxide electrochemical cells, Electrochem. Mater. Technol., 2 (2023) 20232025. https://doi.org/10.15826/elmattech.2023.2.025
Ndubuisi A, Abouali S, Singh K, Thangadurai V, Recent advances, practical challenges, and perspectives of intermediate temperature solid oxide fuel cell cathodes, J. Mater. Chem. A, 10 (2022) 2196–2227. https://doi.org/10.1039/D1TA08475E
Ahmad MZ, Ahmad SH, Chen RS, et al., Review on recent advancement in cathode material for lower and intermediate temperature solid oxide fuel cells application, Int. J. Hydrog. Energy, 47(2) (2022) 1103–1120. https://doi.org/10.1016/j.ijhydene.2021.10.094
Sadykov V, Pikalova E, Sadovskaya E, et al., Design of Mixed Ionic-Electronic Materials for Permselective Membranes and Solid Oxide Fuel Cells Based on Their Oxygen and Hydrogen Mobility, Membranes, 13(8) (2023) 698. https://doi.org/10.3390/membranes13080698
Shaikh SPS, Muchtar A, Somalu MR, A review on the selection of anode materials for solid-oxide fuel cells, Renew. Sustain. Energy Rev., 51 (2015) 1–8. https://doi.org/10.1016/j.rser.2015.05.069
Ahmed N, Devi S, Dar MA, et al., Anode material for solid oxide fuel cell: a review, Indian J. Phys., 98 (2024) 877–888. https://doi.org/10.1007/s12648-023-02860-3
Zainon AN, Somalu MR, Kamarul Bahrain AM, et al., Challenges in using perovskite-based anode materials for solid oxide fuel cells with various fuels: a review, Int. J. Hydrog. Energy, 48(53) (2023) 20441–20464. https://doi.org/10.1016/j.ijhydene.2022.12.192
Shu L, Sunarso J, Hashim SS, et al., Advanced perovskite anodes for solid oxide fuel cells: A review, Int. J. Hydrog. Energy, 44(59) (2019) 31275–31304. https://doi.org/10.1016/j.ijhydene.2019.09.220
Osinkin DA, Some aspects of hydrogen oxidation in solid oxide fuel cell: A brief historical overview, Electrochem. Mater. Technol., 2 (2023) 20232018. https://doi.org/10.15826/elmattech.2023.2.018
Khrustov AV, Ananyev MV, Bronin DI, et al., Characterisation of Ni-cermet degradation phenomena II. Relationship between connectivity and resistivity, J. Power Sources, 497 (2021) 229847. https://doi.org/10.1016/j.jpowsour.2021.229847
Chen H, Wang F, Wang W, et al., H2S poisoning effect and ways to improve sulfur tolerance of nickel cermet anodes operating on carbonaceous fuels, Appl. Energy, 179 (2016) 765–777. https://doi.org/10.1016/j.apenergy.2016.07.028
Istomin SYa, Lyskov NV, Mazo GN, Antipov EV, Electrode materials based on complex d-metal oxides for symmetrical solid oxide fuel cells, Russ. Chem. Rev., 90 (2021) 644–676. https://doi.org/10.1070/RCR4979
Yusoff WNAW, Baharuddin NA, Somalu MR, et al., A Short Review on Selection of Electrodes Materials for Symmetrical Solid Oxide Fuel Cell, IOP Conf. Series: Mater. Sci. Engin., 957 (2020) 012049. https://doi.org/10.1088/1757-899X/957/1/012049
Arregui A, Rodriguez-Martinez LM, Modena S, et al., Stability of ferritic perovskite cathodes in anode-supported solid oxide fuel cells under different processing and operation parameters, Electrochim. Acta, 58 (2011) 312–321. https://doi.org/10.1016/j.electacta.2011.09.048
Xu X, Su C, Shao Z, Fundamental Understanding and Application of Ba0.5Sr0.5Co0.8Fe0.2O3-δ Perovskite in Energy Storage and Conversion: Past, Present, and Future, Energy Fuels, 35(17) (2021) 13585–13609. https://doi.org/10.1021/acs.energyfuels.1c02111
Fernández-Ropero AJ, Porras-Vázquez JM, Cabeza A, et al., High valence transition metal doped strontium ferrites for electrode materials in symmetrical SOFCs, J. Power Sources, 249 (2014) 405–413. https://doi.org/10.1016/j.jpowsour.2013.10.118
Liu F, Zhang L, Huang G, et al., High performance ferrite–based anode La0.5Sr0.5Fe0.9Mo0.1O3-δ for intermediate–temperature solid oxide fuel cell, Electrochim. Acta, 255 (2017) 118–126. https://doi.org/10.1016/j.electacta.2017.09.157
Yang X, Li R, Yang Y, et al., Improving stability and electrochemical performance of Ba0.5Sr0.5Co0.2Fe0.8O3-δ electrode for symmetrical solid oxide fuel cells by Mo doping, J. Alloys Compd., 831 (2020) 154711. https://doi.org/10.1016/j.jallcom.2020.154711
Teketel BS, Beshiwork BA, Desta HG, et al., Ta/Nb doping motivating cubic phase stability and CO2 tolerance of Sr2Co1.6Fe0.4O6-δ double perovskite for high-performing SOFC cathode, Ceram. Int., 50(7A) (2024) 11246–11258. https://doi.org/10.1016/j.ceramint.2024.01.025
Li D, Zhang X, Jin Y, et al., Suppression of Sr surface segregation in La0.8Sr0.2Co0.2Fe0.8O3-δ via Nb doping in B-site, Ceram. Int., 48(2) (2022) 2161–2168. https://doi.org/10.1016/j.ceramint.2021.09.305
Chivite-Lacaba M, Prado-Gonjal J, Alonso JA, et al., High performance of SrCo1-xZrxO3-δ perovskite cathodes for IT-SOFCs, Ceram. Int., 50(15) (2024) 26929–26937. https://doi.org/10.1016/j.ceramint.2024.04.424
Zamudio-García J, Caizán-Juanarena L, Porras-Vázquez JM, et al., A review on recent advances and trends in symmetrical electrodes for solid oxide cells, J. Power Sources, 520 (2022) 230852. https://doi.org/10.1016/j.jpowsour.2021.230852
Meng X, Lü S, Ji Y, et al., Characterization of
Pr1-xSrxCo0.8Fe0.2O3-δ (0.2≤x≤0.6) cathode materials for intermediate-temperature solid oxide fuel cells, J. Power Sources, 183(2) (2008) 581–585. https://doi.org/10.1016/j.jpowsour.2008.05.052
Patro PK, Delahaye T, Bouyer E, Development of Pr0.58Sr0.4Fe0.8Co0.2O3-δ-GDC composite cathode for solid oxide fuel cell (SOFC) application, Solid State Ion., 181(29–30) (2010) 1378–1386. https://doi.org/10.1016/j.ssi.2010.07.004
Liu X, Li M, Wang Z, et al., Electro-spinning Pr0.4Sr0.6Co0.2Fe0.7Nb0.1O3-δ nanofibers infiltrated with Gd0.2Ce0.8O1.9 nanoparticles as cathode for intermediate temperature solid oxide fuel cell, Ceram. Int., 42(10) (2016) 11907–11912. https://doi.org/10.1016/j.ceramint.2016.04.113
Kim JH, Baek S-W, Lee C, et al., Performance analysis of cobalt-based cathode materials for solid oxide fuel cell, Solid State Ion., 179(27–32) (2008) 1490–1496. https://doi.org/10.1016/j.ssi.2008.01.086
Yang C, Yang Z, Jin C, et al., Sulfur‐Tolerant Redox‐Reversible Anode Material for Direct Hydrocarbon Solid Oxide Fuel Cells, Adv. Mater., 24(11) (2012) 1439–1443. https://doi.org/10.1002/adma.201104852
Zhang P, Guan G, Khaerudini DS, et al., Properties of A-site nonstoichiometry (Pr0.4)Sr0.6Co0.2Fe0.7Nb0.1O3-δ (0.9 ≤ x ≤ 1.1) as symmetrical electrode material for solid oxide fuel cells, J. Power Sources, 248 (2014) 163–171. https://doi.org/10.1016/j.jpowsour.2013.09.077
Xiaokaiti P, Yu T, Yoshida A, et al., Effects of cobalt and iron proportions in Pr0.4Sr0.6Co0.9-xFexNb0.1O3-δ electrode material for symmetric solid oxide fuel cells, J. Alloys Compd., 831 (2020) 154738. https://doi.org/10.1016/j.jallcom.2020.154738
Li P, Dong R, Wang Y, et al., Improving the performance of Pr0.4Sr0.6Co0.2Fe0.7Nb0.1O3-δ-based single-component fuel cell and reversible single-component cells by manufacturing A-site deficiency, Renew. Energy, 177 (2021) 387–396. https://doi.org/10.1016/j.renene.2021.05.141
Partovi K, Geppert B, Liang F, et al., Effect of the B-Site Composition on the Oxygen Permeability and the CO2 Stability of Pr0.6Sr0.4CoxFe1-xO3-δ (0.0 ≤ x ≤ 1.0) Membranes, Chem. Mater, 27(8) (2015) 2911–2919. https://doi.org/10.1021/acs.chemmater.5b00166
Zhao Z, Rehder L, Steinbach F, Feldhoff A, High-Entropy Perovskites Pr1-xSrx(Cr,Mn,Fe,Co,Ni)O3-δ (x=0–0.5): Synthesis and Oxygen Permeation Properties, Membranes, 12(11) (2022) 1123. https://doi.org/10.3390/membranes12111123
Ishihara T, Kudo T, Matsuda H, Takita Y, Doped PrMnO3 Perovskite Oxide as a New Cathode of Solid Oxide Fuel Cells for Low Temperature Operation, J. Electrochem. Soc., 142(5) (1995) 1519–1524. https://doi.org/10.1149/1.2048606
Zhang P, Guan G, Khaerudini DS, et al., Mo doped Pr0.4Sr0.6Co0.2Fe0.8O3-δ cathode material with high catalytic activity for intermediate-temperature solid oxide fuel cells, Electrochim. Acta, 146 (2014) 591–597. https://doi.org/10.1016/j.electacta.2014.08.154
Gordeev EV, Porotnikova NM, Approaches for the preparation of dense ceramics and sintering aids for Sr/Mg doped lanthanum gallate: focus review, Electrochem. Mater. Technol., 2 (2023) 20232022. https://doi.org/10.15826/elmattech.2023.2.022
Gorelov VP, Bronin DI, Sokolova JuV, et al., The effect of doping and processing conditions on properties of
La1-xSrxGa1-yMgyO3-α, J. Eur. Ceram. Soc., 21(13) (2001) 2311–2317. https://doi.org/10.1016/S0955-2219(01)00203-5
Osinkin DA, Complementary effect of ceria on the hydrogen oxidation kinetics on Ni-Ce0.8Sm0.2O2-δ anode, Electrochim. Acta, 330 (2020) 135257. https://doi.org/10.1016/j.electacta.2019.135257
Osinkin DA, An approach to the analysis of the impedance spectra of solid oxide fuel cell using the DRT technique, Electrochim. Acta, 372 (2021) 137858. https://doi.org/10.1016/j.electacta.2021.137858
Xiaokaiti P, Yu T, Yoshida A, et al., Characterization of B‐Site Niobium‐Doped Pr0.4Sr0.6(Co0.3Fe0.6)1‐xNbxO3‐δ (x=0, 0.05, 0.1, 0.2) Perovskites as Cathode Materials for Solid Oxide Fuel Cells, ChemistrySelect, 3(17) (2018) 4609–4618. https://doi.org/10.1002/slct.201702180
Patrakeev MV, Mitberg EB, Lakhtin AA, et al., Oxygen Nonstoichiometry, Conductivity, and Seebeck Coefficient of La0.3Sr0.7Fe1-xGaxO2.65+δ Perovskites, J. Solid State Chem., 167(1) (2002) 203–213. https://doi.org/10.1006/jssc.2002.9644
Rietveld HM, A profile refinement method for nuclear and magnetic structures, J. Appl. Crystallogr., 2 (1969) 65–71. https://doi.org/10.1107/S0021889869006558
Zhang P, Guan G, Khaerudini DS, et al., B-site Mo-doped perovskite Pr0.4Sr0.6(Co0.2Fe0.8)1-xMoxO3-δ (x = 0, 0.05, 0.1 and 0.2) as electrode for symmetrical solid oxide fuel cell. J. Power Sources, 276 (2015) 347–356. https://doi.org/10.1016/j.jpowsour.2014.11.141
Lan R, Cowin PI, Sengodan S, Tao S, A perovskite oxide with high conductivities in both air and reducing atmosphere for use as electrode for solid oxide fuel cells, Sci. Rep., 6 (2016) 31839. https://doi.org/10.1038/srep31839
Osinkin DA, Antonova EP, Shubin KS, Bogdanovich NM, Influence of nickel exsolution on the electrochemical performance and rate-determining stages of hydrogen oxidation on Sr1.95Fe1.4Ni0.1Mo0.5O6-δ promising electrode for solid state electrochemical devices, Electrochim. Acta, 369 (2021) 137673. https://doi.org/10.1016/j.electacta.2020.137673
Zhang D, Zhang K, He T, et al., Preparation and characterization of a redox-stable Pr0.4Sr0.6Fe0.875Mo0.125O3-δ material as a novel symmetrical electrode for solid oxide cell application, Int. J. Hydrog. Energy, 45(41) (2020) 21825–21835. https://doi.org/10.1016/j.ijhydene.2020.05.206
Li Z, Wang J, Zhu J, et al., A novel Ba0.95La0.05Fe0.9Nb0.1O3−δ ceramic electrode for symmetrical solid oxide fuel cells. Sustain, Energy Fuels, 5 (2021) 5975–5984. https://doi.org/10.1039/D1SE01291F
Wu Y, Yang Y, Zhou S, et al., Enhanced redox-stable Sm0.5Sr0.5FeO3-δ electrode material for symmetric solid oxide fuel cells at reduced temperatures, Ceram. Int., 46(5) (2020) 6714–6722. https://doi.org/10.1016/j.ceramint.2019.11.160
Filonova EA, Dmitriev AS, Pikalov PS, et al., The structural and electrical properties of Sr2Ni0.75Mg0.25MoO6 and its compatibility with solid state electrolytes, Solid State Ion., 262 (2014) 365–369. https://doi.org/10.1016/j.ssi.2013.11.036
Zhao L, Chen K, Liu Y, He B, A novel layered perovskite as symmetric electrode for direct hydrocarbon solid oxide fuel cells, J. Power Sources, 342 (2017) 313–319. https://doi.org/10.1016/j.jpowsour.2016.12.066
Niu B, Jin F, Feng T, et al., A-site deficient (La0.6Sr0.4)1-xCo0.2Fe0.6Nb0.2O3-δ symmetrical electrode materials for solid oxide fuel cells, Electrochim. Acta, 270 (2018) 174–182. https://doi.org/10.1016/j.electacta.2018.03.085
Tao S, Irvine JTS, A redox-stable efficient anode for solid-oxide fuel cells, Nat. Mater., 2 (2003) 320–323. https://doi.org/10.1038/nmat871
Osinkin DA, Hydrogen oxidation kinetics on a redox stable electrode for reversible solid-state electrochemical devices: The critical influence of hydrogen dissociation on the electrode surface, Electrochim. Acta, 389 (2021) 138792. https://doi.org/10.1016/j.electacta.2021.138792
Jiang SP, A review of wet impregnation—An alternative method for the fabrication of high performance and nano-structured electrodes of solid oxide fuel cells, Mater. Sci. Eng. A, 418(1–2) (2006) 199–210. https://doi.org/10.1016/j.msea.2005.11.052
Ding D, Li X, Lai SY, et al., Enhancing SOFC cathode performance by surface modification through infiltration, Energy Environ. Sci., 7 (2014) 552. https://doi.org/10.1039/c3ee42926a
Filonova E, Pikalova E, Overview of Approaches to Increase the Electrochemical Activity of Conventional Perovskite Air Electrodes, Materials, 16(14) (2023) 4967. https://doi.org/10.3390/ma16144967
Osinkin DA, Bogdanovich NM, Gavrilyuk AL, Rate determining steps of fuel oxidation over CeO2 impregnated Ni-YSZ in H2+H2O+CO+CO2 ambient, Electrochim. Acta, 199 (2016) 108–115. https://doi.org/10.1016/j.electacta.2016.03.133
Osinkin DA, Bogdanovich NM, Beresnev SM, Zhuravlev VD, High-performance anode-supported solid oxide fuel cell with impregnated electrodes, J. Power Sources, 288 (2015) 20–25. https://doi.org/10.1016/j.jpowsour.2015.04.098
Yang Z, Wang W, Xiao J, et al., A novel cobalt-free Ba0.5Sr0.5Fe0.9Mo0.1O3−δ–BaZr0.1Ce0.7Y0.2O3-α composite cathode for solid oxide fuel cells, J. Power Sources, 204 (2012) 89–93. https://doi.org/10.1016/j.jpowsour.2012.01.044
Yang Z, Xu N, Han M, Chen F, Performance evaluation of La0.4Sr0.6Co0.2Fe0.7Nb0.1O3-δ as both anode and cathode material in solid oxide fuel cells, Int. J. Hydrog. Energy, 39(14) (2014) 7402–7406. https://doi.org/10.1016/j.ijhydene.2014.01.009
Pikalova EYu, Maragou VI, Demina AN, et al., The effect of co-dopant addition on the properties of Ln0.2Ce0.8O2-δ (Ln=Gd, Sm, La) solid-state electrolyte, J. Power Sources, 181(2) (2008) 199–206. https://doi.org/10.1016/j.jpowsour.2008.02.003
DOI: https://doi.org/10.15826/elmattech.2024.3.039
Copyright (c) 2024 Denis A. Osinkin, Elena Yu. Pikalova

This work is licensed under a Creative Commons Attribution 4.0 International License.

