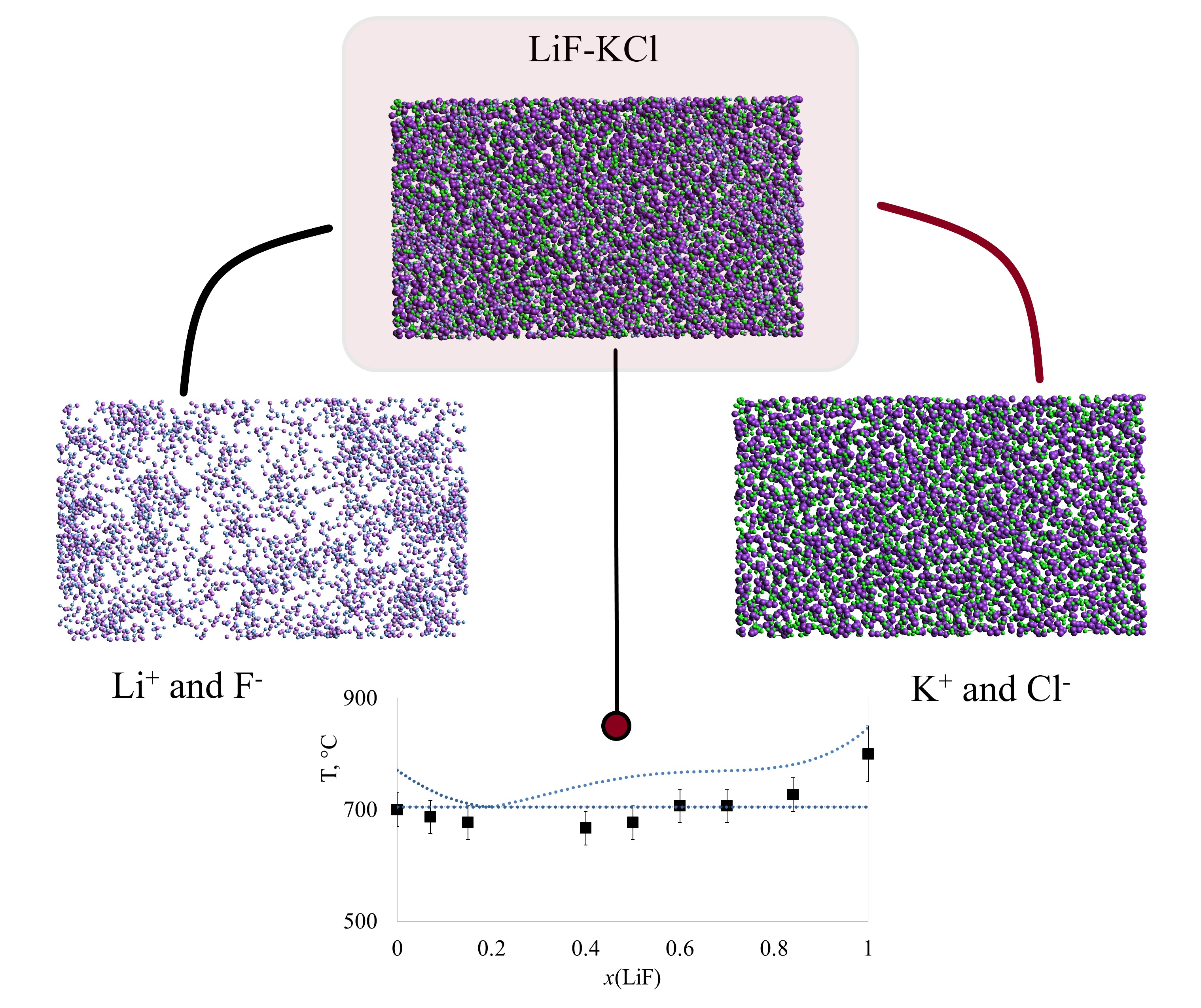
Revealing the effect of local structure on phase equilibria in binary molten salts
Abstract
Keywords
References
Galwey AK, A view and a review of the melting of alkali metal halide crystals. Part 2. Pattern of eutectics and solid solutions in binary common ion mixtures, J. Therm. Anal. Calorim., 82 (2005) 423–437. https://doi.org/10.1007/s10973-005-0913-1
Oonk HAJ, Solid-state solubility and its limits. The alkali halide case, Pure Appl. Chem., 73(5) (2001) 807–823. https://doi.org/10.1351/pac200173050807
van der Kemp WJM, Blok JG, van Genderen ACG, van Ekeren PJ, et. al., Binary common-ion alkali halide mixtures; a uniform description of the liquid and solid state, Thermochim. Acta, 196(2) (1992) 301–315. https://doi.org/10.1016/0040-6031(92)80093-C
Garkushin IK, Burchakov AV, Emelyanova UA, Chugunova MV, Phase complex of the quinary reciprocal system Li+, Na+, K+ || F-, Cl-, Br- and investigation of the stable tetrahedron LiF-NaF-KCl-KBr, Russ. J. Inorg. Chem., 65 (2020) 1040–1046. https://doi.org/10.1134/S0036023620070086
Burns JH, Busing WR, Crystal structures of rubidium lithium fluoride, RbLiF2, and cesium lithium fluoride, CsLiF2, Inorg. Chem., 4(10) (1965) 1510–1512. https://doi.org/10.1021/ic50032a041
Pentin IV, Schön JC, Jansen M, Ab initio prediction of the low-temperature phase diagrams in the systems CsX-LiX (X=F, Cl, Br, I), Solid State Sci., 10(6) (2008) 804–813. https://doi.org/10.1016/j.solidstatesciences.2007.06.001
Lipkina K, Palinka K, Geiger E, Fitzpatrick BWN, et. al., Thermodynamic investigations of the LiF-CsF and NaF-CsF pseudo-binary systems, J. Nucl. Mater., 568 (2022) 153901. https://doi.org/10.1016/j.jnucmat.2022.153901
Sprouster D, Zheng G, Lee SC, Olds D, et. al., Molecular structure and phase equilibria of molten fluoride salt with and without dissolved cesium: FLiNaK-CsF (5 mol %), ACS Appl. Energy Mater., 5(7) (2022) 8067–8074. https://doi.org/10.1021/acsaem.2c00544
Margheritis C, Flor G, Sinistri C, Miscibility gaps in fused salts VII. Systems of LiF with alkali halides, Z. Naturforsch. A, 28 (1973) 1329–1334. https://doi.org/10.1515/zna-1973-0813
Stepanov VP, Babushkina LM, Dokashenko SI, Liquid + liquid equilibrium in mixtures of lithium fluoride with potassium and rubidium halides, J. Chem. Thermodyn., 51 (2012) 12–16. https://doi.org/10.1016/j.jct.2012.02.015
Stepanov VP, Density of separating salt melts in the two-phase region, Russ. Metall., 2022 (2022) 830–836. https://doi.org/10.1134/S0036029522080171
Tkachev NK, Rukavishnikova IV, Lokett VN, Stepanov VP, Density of stratified ionic melts: Experiments and theory, Russ. J. Electrochem., 43 (2007) 955–960. https://doi.org/10.1134/S1023193507080149
Blander M, Topol LE, The topology of phase diagrams of reciprocal molten salt systems, Inorg. Chem., 5(10) (1966) 1641–1645. https://doi.org/10.1021/ic50044a002
Xiao T, Zhou Y, Jiang H, Surface tension of simple molten salts: Insight from a charged hard sphere model, J. Phys. Chem. B, 128(32) (2024) 7882–7887. https://doi.org/10.1021/acs.jpcb.4c03118
Davydov AG, Tkachev NK, Heat capacity of molten alkali halides, J. Mol. Liq., 356 (2022) 119032. https://doi.org/10.1016/j.molliq.2022.119032
Vega C, Bresme F, Abascal JLF, Fluid-solid equilibrium of a charged hard-sphere model, Phys. Rev. E, 54 (1996) 2746. https://doi.org/10.1103/PhysRevE.54.2746
Dixon M, Gillan MJ, Structure of molten alkali chlorides I. A molecular dynamics study, Phil. Mag. B, 43(6) (1981) 1099–1112. https://doi.org/10.1080/01418638108222577
Adams DJ, McDonald IR, Rigid-ion models of the interionic potential in the alkali halides, J. Phys. C: Solid State Phys., 7(16) (1974) 2761–2775. https://doi.org/10.1088/0022-3719/7/16/009
Wang J, Sun Z, Lu G, Yu J, Molecular dynamics simulations of the local structures and transport coefficients of molten alkali chlorides, J. Phys. Chem. B, 118(34) (2014) 10196–10206. https://doi.org/10.1021/jp5050332
Ribeiro MCC, Chemla effect in molten LiCl/KCl and LiF/KF mixtures, J. Phys. Chem. B, 107(18) (2003) 4392–4402. https://doi.org/10.1021/jp027261a
Salanne M, Simon C, Turq P, Madden PA, Intermediate range chemical ordering of cations in simple molten alkali halides, J. Phys.: Condens. Matter, 20 (2008) 332101. https://doi.org/10.1088/0953-8984/20/33/332101
Shao J, Shu G, Xu H, Chen N, Molecular dynamics calculation of molten LiF, KCl and LiF-KCl (1:1) system, Chinese Phys. Lett., 6 (1989) 448–450. https://doi.org/10.1088/0256-307X/6/10/005
Kobelev MA, Tatarinov AS, Zakiryanov DO, Tkachev NK, Calculation of liquidus curve in phase diagram LiCl-KCl by molecular dynamics simulation, Phase Transitions, 93(5) (2020) 504–508. https://doi.org/10.1080/01411594.2020.1758318
Bin Faheem A, Lee KK, Development of a neural network potential for modeling molten LiCl/KCl salts: Bridging efficiency and accuracy, J. Phys. Chem. C, 128(5) (2024) 2163–2178. https://doi.org/10.1021/acs.jpcc.3c07010
DeFever RS, Maginn EJ, Computing the liquidus of binary monatomic salt mixtures with direct simulation and alchemical free energy methods, J. Phys. Chem. A, 125(38) (2021) 8498–8513. https://doi.org/10.1021/acs.jpca.1c06107
Haendler HM, Sennett PS, Wheeler CM, The system LiF-LiCl, LiF-NaCl, LiF-KCl, J. Electrochem. Soc., 106 (1959) 264–268. https://doi.org/10.1149/1.2427319
Chartrand P, Pelton AD, Thermodynamic evaluation and optimization of the Li, Na, K, Mg, Ca // F, Cl reciprocal system using the modified quasi-chemical model, Metall. Mater. Trans. A, 32 (2001) 1417–1430. https://doi.org/10.1007/s11661-001-0231-6
Zakiryanov D, Kobelev M, Tkachev N, Melting properties of alkali halides and the cation-anion size difference: A molecular dynamics study, Fluid Phase Equil., 506 (2020) 112369. https://doi.org/10.1016/j.fluid.2019.112369
DOI: https://doi.org/10.15826/elmattech.2024.3.045
Copyright (c) 2024 Mikhail A. Kobelev

This work is licensed under a Creative Commons Attribution 4.0 International License.

