Ln2NiO4+δ-based oxygen electrodes for proton-conducting Sr0.98Zr0.95Yb0.05O3–δ electrolyte for application in IT-SOFCs
Abstract
Keywords
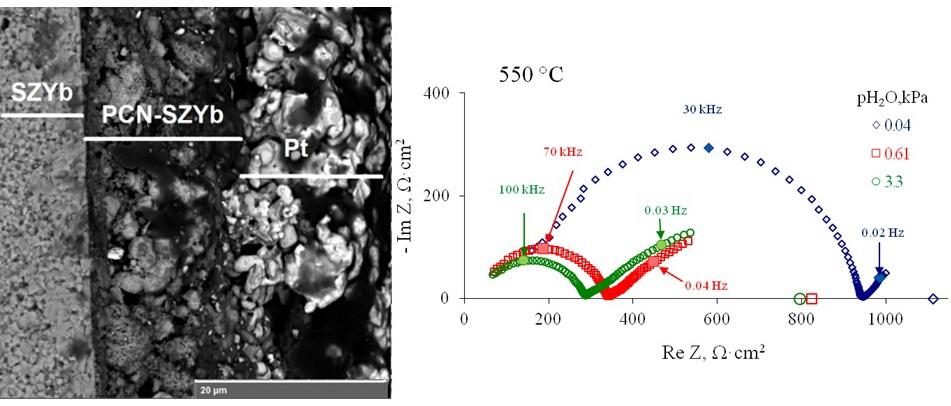
Full Text:
PDFReferences
Xu Q, Guo Z, Xia L, He Q, et al., A comprehensive review of solid oxide fuel cells operating on various promising alternative fuels, Energy Conversion and Management, 253 (2022) 115175. https://doi.org/10.1016/j.enconman.2021.115175
Dawood F, Anda M, Shafiullah GM, Hydrogen production for energy: an overview, Int. J. Hydrogen Energy, 45 (2020) 3847–3869. https://doi.org/10.1016/j.ijhydene.2019.12.059
Choi S, Davenport TC, Haile SM, Protonic ceramic electrochemical cells for hydrogen production and electricity generation: Exceptional reversibility, stability, and demonstrated faradaic efficiency, Energy Environ. Sci., 12 (2019) 206–215. https://doi.org/10.1039/C8EE02865F
Duan C, Huang J, Sullivan N, O’Hayre R, Proton-conducting oxides for energy conversion and storage, Appl. Phys. Rev., 7 (2020) 011314. https://doi.org/10.1063/1.5135319
Zhang W, Hu YH, Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices, Energy Sci. Eng., 9 (2021) 984–1011. https://doi.org/10.1002/ese3.886
Yajima T, Suzuki H, Yogo T, Iwahara H, Protonic conduction in SrZrO3-based oxides. Solid State Ionics, 51 (1992) 101–107. https://doi.org/10.1016/0167-2738(92)90351-O
Zajac W, Rusinek D, Zheng K, Molenda J, Applicability of Gd-doped BaZrO3, SrZrO3, BaCeO3 and SrCeO3 proton conducting perovskites as electrolytes for solid oxide fuel cells, Cent. Eur. J. Chem., 11 (2013) 471–484. https://doi.org/10.2478/s11532-012-0144-9
Gharbage B, Marques FMB, Frade JR, Protonic conduction in Sr1-y(Zr1-xDyx)O3-d ceramics, J. Eur. Ceram. Soc., 16 (1996) 1149–1156. https://doi.org/10.1016/0955-2219(96)00052-0
Higuchi T, Tsukamoto T, Sata N, Hiramoto K, et al., Protonic conduction in the single crystals of SrZr0.95M0.05O3 (M = Y, Sc, Yb, Er), Jpn. J. Appl. Phys., 40 (2001) 4162–4163. https://doi.org/10.1143/JJAP.40.4162
Dunyushkina LA, Khaliullina ASh, Meshcherskikh AN, Pankratov AA, et al., Effect of A-site nonstoichiometry on defect chemistry and electrical conductivity of undoped and Y-doped SrZrO3, Materials, 12 (2019) 1258. https://doi.org/10.3390/ma12081258
Shkerin SN, Rudakova AV, Bulanin KM, Khaliullina ASh, et al., Raman spectroscopy of SrZrO3 based proton conducting electrolyte: Effect of Y-doping and Sr-nonstoichiometry, Int. J. Hydrogen Energy, 46 (2021) 17007–17018. https://doi.org/10.1016/j.ijhydene.2020.11.236
Balakireva VB, Gorelov VP, Dunyushkina LA, Kuzmin AV, Impact of Humidity on Charge Transport in Proton-Conducting Perovskites AZr0.95Sc0.05O3-d (A = Ca, Sr, Ba) Exposed to an Oxidative Atmosphere, Phys. Solid State, 61 (2019) 515–522. https://doi.org/10.1134/S1063783419040048
Khaliullina A, Meshcherskikh A, Pankratov A, Dunyushkina L, Effect of Sr deficiency on electrical conductivity of Yb-doped strontium zirconate, Materials, 15 (2022) 4126. https://doi.org/10.3390/ma15124126
Khaliullina A, Meshcherskikh A, Dunyushkina L, Effect of cation nonstoichiometry on hydration and charge transport processes in Yb-doped SrZrO3 perovskite-type proton conductor for ceramic electrochemical cells, Processes, 11 (2023) 2939. https://doi.org/10.3390/pr11102939
Sadykov VA, Muzykantov VS, Yeremeev NF, PelipenkoVV, et al., Solid Oxide Fuel Cell Cathodes: Importance of Chemical Composition and Morphology, Catalysis for Sustainable Energy, 2(1) (2015) 57–70. https://doi.org/10.1515/cse-2015-0004
Gao Y, Zhang M, Fu M, Hu W, et al., A comprehensive review of recent progresses in cathode materials for proton-conducting SOFCs, Energy Reviews, 2(3) (2023) 100038, https://doi.org/10.1016/j.enrev.2023.100038
Song J, Ning D, Boukamp B, Bassat JM, et al., Structure, electrical conductivity and oxygen transport properties of Ruddlesden–Popper phases Lnn+1NinO3n+1 (Ln = La, Pr and Nd; n = 1, 2 and 3), J. Mater. Chem. A, 8 (2020) 22206. https://doi.org/10.1039/d0ta06731h
Morales-Zapata MA, Larrea A, Laguna-Bercero MA, Lanthanide nickelates for their application on Solid Oxide Cells, Electrochimica Acta, 444 (2023) 141970. https://doi.org/10.1016/j.electacta.2023.141970
Osaka T, Numako C, Koto K, Local structure and thermal study of ytterbium-doped SrZrO3, Materials Research Bulletin, 34 (1999) 11–24. https://doi.org/10.1016/S0025-5408(98)00209-8
Greenblatt M, Ruddlesden-Popper properties Lnn+1NinO3n+1 nickelates: structure and properties, Curr. Opin. Solid State Mater. Sci., 2 (1997) 174–183. https://doi.org/10.1016/s1359-0286(97)80062-9
Kharton VV, Viskup AP, Naumovich EN, Marques FMB, Oxygen ion transport in La2NiO4-based ceramics, J. Mater., 9 (1999) 2623–2629. https://doi.org/10.1039/A903276B
Skinner SJ, Kilner JA, Oxygen diffusion and surface exchange in La2-xSrxNiO4+δ, Solid State Ionics 135 (2000) 709–712. https://doi.org/10.1016/S0167-2738(00)00388-X
Bassat JM, Odier P, Villesuzanne A, Marin C, et al., Anisotropic ionic transport properties in La2NiO4+δ single crystals, Solid State Ionics, 167 (2004) 341–347. https://doi.org/10.1016/j.ssi.2003.12.012
Lee Y, Kim H, Electrochemical performance of La2NiO4+δ cathode for intermediate-temperature solid oxide fuel cells, Ceram. Int., 41 (2015) 5984–5991. https://doi.org/10.1016/j.ceramint.2015.01.037
Nicollet C, Flura A, Vibhu V, Rougieret A, et al., La2NiO4+δ infiltrated into gadolinium doped ceria as novel solid oxide fuel cell cathodes: electrochemical performance and impedance modeling, J. Power Sources, 294 (2015) 473–482. https://doi.org/10.1016/j.jpowsour.2015.06.077
Woolley RJ, Skinner SJ, Functionally graded composite La2NiO4+δ and La4Ni3O10−δ solid oxide fuel cell cathodes, Solid State Ionics, 255 (2014) 1–5. https://doi.org/10.1016/j.ssi.2013.11.041
Zhao K, Wang Y-P, Chen M, Xu Q, et al., Electrochemical evaluation of La2NiO4+δ as a cathode material for intermediate temperature solid oxide fuel cells, Int. J. Hydrog. Energy, 39 (2014) 7120–7130. https://doi.org/10.1016/j.ijhydene.2014.02.106
Kolchugin AА, Pikalova EY, Bogdanovich NM, Bronin DI, et al., Structural, electrical and electrochemical properties of calcium-doped lanthanum nickelate, Solid State Ionics, 288 (2016) 48–53. https://doi.org/10.1016/j.ssi.2016.01.035
Tarutin A, Lyagaeva J, Farlenkov A, Plaksin S, et al., Reversible Protonic Ceramic Cell with Symmetrically Designed Pr2NiO4+δ-Based Electrodes: Fabrication and Electrochemical Features, Materials, 12(1) (2019) 118. https://doi.org/10.3390/ma12010118
Hyegsoon A, Dongwook S, Ho-Il J, Pr2NiO4+δ for Cathode in Protonic Ceramic Fuel Cells, J. Korean Ceram. Soc., 55(4) (2018) 358–363. https://doi.org/10.4191/kcers.2018.55.4.06
Grimaud A, Mauvy F, Bassat JM, Fourcade S, et al., Hydration and transport properties of the Pr2−xSrxNiO4+δ compounds as H+-SOFC cathodes, J. Mater. Chem., 22 (2012) 16017–16025 https://doi.org/10.1039/C2JM31812A
Pikalova E, Zhulanova T, Ivanova A, Tarutin A, et al., Optimized Pr1.6Ca0.4Ni1−yCuyO4+δ phases as promising electrode materials for CeO2- and BaCe(Zr)O3-based electrochemical cells, Ceramics International, 50(20) Part C (2024) 40476–40491. https://doi.org/10.1016/j.ceramint.2024.06.048
Yang X, Xu X, Wu S, Yu S, et al., Enhancing the performance of traditional La2NiO4+δ cathode for proton-conducting solid oxide fuel cells with Zn-doping, Ceramics International, 48(14) (2022) 19626–19632. https://doi.org/10.1016/j.ceramint.2022.03.098
Pikalova EY, Kolchugin AA, The Influence of the Substituting Element (M = Ca, Sr, Ba) in La1.7M0.3NiO4+δ on the Electrochemical Performance of the Composite Electrodes, Eurasian Chemico-Technological Journal, 18(1) (2016) 3–11. https://doi.org/10.18321/ectj386
Antonova EP, Kolchugin AA, Pikalova EY, Medvedev DA, et al., Development of electrochemically active electrodes for BaCe0.89Gd0.1Cu0.01O3−δ proton conducting electrolyte, Solid State Ionics, 306 (2017) 55–61. https://doi.org/10.1016/j.ssi.2017.02.001
Danilov N, Lyagaeva J, Vdovin G, Pikalova E, et al., Electricity/hydrogen conversion by the means of a protonic ceramic electrolysis cell with Nd2NiO4+δ-based oxygen electrode, Energy Conversion and Management, 172 (2018) 129–137. https://doi.org/10.1016/j.enconman.2018.07.014
Gilev AR, Sukhanov KS, Kiselev EA, Sobol ME, et al., Increasing thermodynamic stability and electrochemical performance of IT-SOFC cathodes based on Ln2MO4 (Ln = La, Pr; M = Ni, Cu), Ceramics International, 50(20) Part C (2024) 40453–40463. https://doi.org/10.1016/j.ceramint.2024.04.176
Solis C, Navarrete L, Serra JM, Study of Pr and Pr and Co doped La2NiO4+δ as cathodes for La5.5WO11.25−δ based protonic conducting fuel cells, Journal of Power Sources, 240 (2013) 691–697. https://doi.org/10.1016/j.jpowsour.2013.05.055
Pikalova EYu, Bogdanovich NM, Kuz’min AV, Composite electrodes for proton conducting electrolyte of CaZr0.95Sc0.05O3–δ, Russian Journal of Electrochemistry, 53(7) (2017) 846–855. https://doi.org/10.1134/S1023193517070096
Antonova EP, Stroeva AY, Tropin ES, Electrode performance of La2NiO4+δ cathodes in contact with La0.9Sr0.1ScO3−δ proton-conducting oxide, J. Solid State Electrochem., 24 (2020) 1447–1451. https://doi.org/10.1007/s10008-020-04535-z
Oh S, Kim H, Jeong I, Kim D, et al., Recent progress in oxygen electrodes for protonic ceramic electrochemical cells, J. Korean Ceram. Soc., 61 (2024) 224–249. https://doi.org/10.1007/s43207-023-00360-y
Vshivkova AI, Gorelov VP, Kuzmin AV, Plaksin SV, et al., Preparation and physicochemical properties of praseodymium oxide films and ceramics, Inorganic Materials, 51(11) 2015 1168–1176. https://doi.org/10.1134/S0020168515100179
Vashook V, Girdauskaite E, Zosel J, Wen TL, et al., Oxygen non-stoichiometry and electrical conductivity of Pr2−xSrxNiO4±δ with x=0–0.5, Solid State Ionics, 177(13–14) (2006) 1163–1171. https://doi.org/10.1016/j.ssi.2006.05.018
Pikalova E, Kolchugin A, Filonova E, Bogdanovich N, et al., Validation of calcium-doped neodymium nickelates as SOFC air electrode materials, Solid State Ionics, 319 (2018) 130–140. https://doi.org/10.1016/j.ssi.2018.02.008
Pikalova E, Sadykov V, Sadovskaya E, Yeremeev N, et al., Correlation between Structural and Transport Properties of Ca-Doped La Nickelates and Their Electrochemical Performance, Crystals, 11(3) (2021) 297. https://doi.org/10.3390/cryst11030297
Nakamura T, Yashiro K, Sato K, Mizusaki J, Electrical conductivity, Seebeck coefficient, and defect structure of oxygen nonstoichiometric Nd2−xSrxNiO4+δ, Materials Chemistry and Physics, 122(1) (2010) 250–258. https://doi.org/10.1016/j.matchemphys.2010.02.044
Nakamura T, Yashiro K, Sato K, Mizusaki J, Electronic state of oxygen nonstoichiometric La2−xSrxNiO4+δ at high temperatures, Phys. Chem. Chem. Phys., 11 (2009) 3055–3062. https://doi.org/10.1039/B823364K
Taherparvar H, Kilner JA, Baker RT, Sahibzada M, Effect of Humidification at Anode and Cathode in Proton-Conducting SOFCs, Solid State Ionics, 162–163 (2003) 297–303. https://doi.org/10.1016/S0167-2738(03)00222-4
Dong K, Miao L, Hou J, Liu W, A novel inhibiting water adsorption strategy to enhance the cathode electrocatalytic ability, Journal of Alloys and Compounds, 876 (2021) 160205. https://doi.org/10.1016/j.jallcom.2021.160205
Kwon Y, Han Y, Fabrication of electrolyte-supported solid oxide fuel cells using a tape casting process, Journal of the Ceramic Society of Japan, 128(6) (2020) 310–316. http://doi.org/10.2109/jcersj2.20006
Pikalova EYu, Bogdanovich NM, Kuzmin AV, Composite electrodes for proton conducting electrolyte of CaZr0.95Sc0.05O3-δ, Russian Journal of Electrochemistry, 53(7) (2017) 752–760. http://doi.org/10.1134/S1023193517070096
Gorelov VP, Balakireva VB, Kuzmin AV, Charge transfer and defect structure in BaCeO3, Russ. J. Inorg. Chem., 63(7) (2018) 930–937. https://doi.org/10.1134/S0036023618070070
Dippon M, Babiniec SM, Ding H, Ricote S, et al., Exploring electronic conduction through BaCexZr0.9-xY0.1O3-δ proton-conducting ceramics, Solid State Ionics, 286 (2016) 117–121. https://doi.org/10.1016/j.ssi.2016.01.029
Kosacki I, Tuller HL, Mixed conductivity in SrCe0.95Yb0.05O3 protonic conductors, Solid State Ionics, 80 (1995) 223–229. https://doi.org/10.1016/0167-2738(95)00142-S
Antonova EP, Kolchugin AA, Pikalova EYu, Medvedev DA, et al., Development of electrochemically active electrodes for BaCe0.89Gd0.1Cu0.01O3-δ proton conducting electrolyte, Solid State Ionics, 306 (2017) 55–61. http://dx.doi.org/10.1016/j.ssi.2017.02.001
DOI: https://doi.org/10.15826/elmattech.2025.4.047
Copyright (c) 2025 Anastasia N. Meshcherskikh, Adelya Sh. Khaliullina, Elena Yu. Pikalova, Liliya A. Dunyushkina

This work is licensed under a Creative Commons Attribution 4.0 International License.

